Psychobiotics as integrative therapy for neuropsychiatric disorders with special emphasis on the microbiota-gut-brain axis.
Magdalini Kavvadia,1§ Gemma Lou De Santis,2§ Nuha Abd Almajeed Abbaas Alwardat,3 Giulia Bigioni,4 Carmen Zeppieri,1 Stefano Cascapera3,5 and Allegra De Lorenzo6
§ Equal contribution.
1 Division of Clinical Nutrition and Nutrigenomics, Department of Biomedicine and prevention, University of Rome "Tor Vergata", Rome, Italy
2 Specialization School of Food Science, University of Rome "Tor Vergata", Rome, Italy
3 PhD School of Applied Medical-Surgical Sciences, University of Rome "Tor Vergata", Rome, Italy
4 Department of Physics, University of Rome "Sapienza", Rome, Italy
5 Iona Preparatory School, New Rochelle, NY, USA
6 Columbia University, New York, USA
Introduction
The human body is colonized by numerous microbial communities residing in both internal and external areas. The total of microorganisms in the intestinal tract is known as the gut microbiota.1 A complex interaction exists between host and gut microflora and this field of research is of growing interest to clinicians and researchers. The gut microbiome consists of the collective genome of about 100 trillion microorganisms residing in the gastrointestinal tract, and contains 150 times as many genes as the human genome.2 Amongst microbial communities, the gut microbiota is considered of particular importance to the host. Humans are in a symbiotic relationship with gut microbes, in which we provide them with a continuous source of nutrition and in return they provide us with health benefits.
For a better understanding of the complex interaction between gut microbiota and the host, and its role in health and disease, a complete description of the microbiome ecology is needed. Both metagenomic and molecular methods have been used for this purpose.3,4 It is estimated that our microbiome consists of more than 1000 species and more than 7000 strains. The human gut is home to 1014-1015 microorganisms, consisting of mainly anaerobic bacteria, but also viruses, protozoa, fungi and archaea.
The gastrointestinal tract of the fetus is considered sterile and gut colonization of the infant begins at birth with the exposure of the newborn to maternal and hospital environment. It is important to note that there is data suggesting the presence of
a prenatal mother-to-child microbiota transmission.5,6 As infants are exposed to a variety of environmental factors, gut microbiota is growing in terms of size and diversity, and around the age of 1 to 2 years an adult-like microbiome is noticeable. The gut microbiome has a dynamic nature and can be influenced by a variety of factors, including route of birth delivery, maternal transfer, genetics, diet, infection, medications such as antibiotics, age and stress.7,8 These factors can alter the composition of the gut transiently, as well as permanently. The gut microbiome is dominated by two bacterial phylotypes, Firmicutes and Bacteroidetes, with other phyla such as Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia present in significantly lower amounts. Although there are interindividual variations in gut microbiota composition, humans have been classified into three ‘enterotypes’, dominated respectively by Prevotella, Ruminococcus and Bacteroides.9
Gut microbiota plays an important role in host physiology. A balanced gut microbial composition could be a marker associated with health, as microbiota dysbiosis is seen, for example, in Irritable Bowel Syndrome (IBS) and Inflammatory Bowel Disease (IBD).10 Possibly, the individual microbiota profile could represent a valuable tool in assessing risk to disease and response to therapy. Gut microbiota lowers the risk of infection by defending against pathogen colonization and protects against bacterial penetration by embattling the intestinal epithelial barrier. These microorganisms contribute also to metabolism by aiding in the breaking down of indigestible fibers and by producing essential metabolites, such as small chain fatty acids (SCFAs). In addition, gut bacteria are the main source of vitamin K and a less vital source of the B complex.11-13 Gut microbiota is a key modulator of the development and function of the host immune system. Indeed, in Germ-Free (GF) mice immune defects are seen at a structural, as well as cellular level.14
Interestingly, gut microbiota is also critical to brain development and function. Bidirectional communication between gut and brain has been long recognized and most of the communicational pathways have been established.15 However, the term microbiota-gut-brain axis is emerging due to a growing body of experimental data focusing on the role of gut microbiome in the brain-gut communication. Moreover, several studies have pointed out the importance of gut microbiota to Central Nervous System (CNS) function,15-17 by being involved in the regulation of neuroinflammation, neuroendocrine stress response, and neurodevelopment. Gut microbiota also has the potential to modulate mood and behavior,1 which implicates it in neurological disorders, such as stress, anxiety, depression and autism that result in disruption of social behavior. Current and future animal and clinical studies dedicated to elucidating the microbiota-gut brain axis will be a breakthrough in preventing and treating mental illness.
There seems to be a balance in the enteric microbiota that confers health benefits and participates in the preservation of homeostasis. Dysbiosis and dysregulation of the microbiota-gut-brain axis have been implicated in gastrointestinal disorders, such as IBD, metabolic, and neurological disorders, such as autism. Luckily, we have the opportunity to target and modulate gut microbiota.14 Emerging research data is suggesting the use of psychobiotics, defined as probiotics that produce health benefits in patients suffering from psychiatric illnesses, or prebiotics as a suitable therapeutic intervention for depression and related disorders.18,19 The bacteria most commonly exploited as probiotics belong to the Bifidobacterium and Lactobacillus families.20 However, more research is required to assess the potential therapeutic effects on patient population.
The focus of this review is the role of gut microbiota in specific neurological disorders and the preventive and therapeutic potential deriving from modulation of gut composition in such psychiatric conditions.
Communication Between Gut Microbiota and Brain
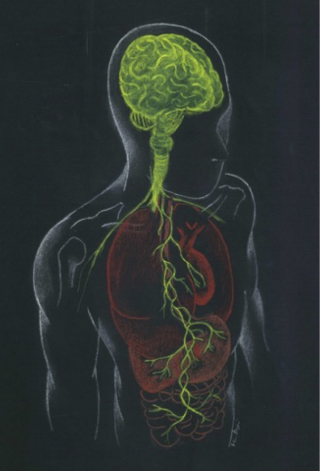
The notion that brain can regulate gastrointestinal activity is well established. A variety of studies conducted both on animals and humans have shown that gut microbiota is critical for normal brain development and function.21-24 In the past decade research has focused on the reverse pathway, how gut microbiota can affect the brain, and what are the mechanisms behind this. The bidirectional communication between the gut and the brain is known as the gut-brain axis. This term has been expanded to microbiota-gut-brain axis, since gut microbiota is of primary importance to this pathway25,26 (Figure 1). The main components of the axis include neural, neuroendocrine, neuroimmune and metabolic pathways.27-29 All of these components interact to form a complex reflex network with afferent fibers projecting to CNS structures for integration and efferent projections to the smooth muscle.30
Figure 1: Representation of the microbiota–gut–brain axis highlighting interactions between enteric microbiota, central and enteric nervous systems.
The CNS communicates with and influences the gut principally through neural and endocrine pathways. The sympathetic division of the autonomic nervous system (ANS) primarily innervates the vascular beds of the gastrointestinal tract (GI) and the enteric nervous system (ENS), and secondarily the lamina propria and Peyer’s patches. The sympathetic nervous system and hypothalamus-pituitary-adrenal axis (HPA) can modulate GI motility, secretion and epithelial permeability.31,32
Gut microbiota can regulate CNS activity through different pathways. The vagus nerve and the ENS are implicated in transmitting the effects of gut microbiota on the brain and vice versa. The majority of vagus nerves (80%) are sensory neurons carrying out information from the gut to the CNS.21,33 Many of the effects of gut microbiota and probiotics have been confirmed as dependent on vagal activation.34 However, vagus independent pathways also exist, as vagotomy has failed to influence certain communicational aspects. Furthermore, gut microbiota can stimulate secretion of cytokines and chemokines that can affect the integrity of the epithelium, and thus elicit an immune response by entering into the systemic circulation. Indirect stimulation of the innate immunity can alter the levels of cytokines, such as interleukin-1 (IL-1) and interleukin-6 (IL-6), which can directly influence the brain by acting on the HPA axis.35 In addition, gut microbiota are important factors in the development and regulation of the HPA axis, which is the major stress response system of our body and is itself involved in the bidirectional communication between brain and gut.36 Finally, gut microbiota can produce neurotransmitters and neuroactive compounds, such as SCFAs that can also modulate brain function.37,38 Alterations in microbiota composition may lead to impairment of the communicational pathways, which can lead to disturbances in the gut-brain axis and ultimately to disease.39Dysregulation of this pathway has been linked to gastrointestinal, as well as stress-related disorders. Modulation of the gut microbiota is revealing new possible therapeutic targets for mood and other disorders.
Gut Microbiota and Stress
Gut microbiota plays an important role in brain development, behavior and mood.1 Moreover, compiling data indicates that intestinal microbes can affect social interaction and stress management (Table 1).
Table 1. Potential beneficial effects of different probiotic species on stress in mice.
Stress is defined as “a state of threatened homeostasis”, and inability to properly respond or adapt to it may lead to disease.43 Indeed, exposure to stress can impair a number of functions and induce GI and mental disorders, such as depression, anxiety, post-traumatic stress disorders and drug addiction.44-46
The HPA axis is the major neuroendocrine stress response system of our body. It consists of the hypothalamus, pituitary gland, adrenal cortex, regulatory inputs and secreted factors and hormones. When activated, cells of the paraventricular nucleus (PVN) release corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP). These stimulate the anterior pituitary gland to secrete adrenocorticotropic hormone (ACTH) into the systemic circulation. ACTH in turn stimulates the adrenal cortex to release cortisol, which has a wide range of functions.47 Apart from glucocorticoids, catecholamines are also released into the circulation. Both psychological and physical stressors can activate the HPA axis. The response to psychological stress is mediated by neurotransmitters, such as serotonin or 5-hydroxytryptamine (5-HT) and norepinephrine (NE). On the other hand, response to physical stressors, for instance to infections, is activated via pro-inflammatory cytokines.48,49
Groundbreaking studies revealed that the gut microbiota is essential to the stress response by being implicated in the development and programming of the HPA axis and in the stress response later in life. They also determined that there is a narrow window of early life colonization that ensures the normal HPA axis development. Sudo et al.36 demonstrated that GF mice exposed to mild restraint stress showed an excessive corticosterone and ACTH release, compared to the specific pathogen free (SPF) controls. This response in GF mice was partly reversed by colonization with SPF animal fecal matter, and fully reversed by association with Bifidobacterium infantis in a time dependent manner. A similar study found that expression of the glucocorticoid receptor in the hippocampus of GF rats was lower than in SPF controls, whereas CRH expression was increased in the hypothalamus of GF animals.50
Both the intestinal microbiota and the stress response develop in parallel during the postnatal period. Therefore, stress, both acute and chronic, and the associated HPA axis can affect gut microbiota composition and function. Most of the related studies have been performed using animal models. Stress can alter intestinal permeability, allowing bacterial translocation across the intestinal mucosa and thus immune system activation, which can have direct effects on CNS.51 Another study showed that pretreatment with the probiotic Lactobacillus farciminis in rats reduced intestinal permeability that results from restraint stress and abated HPA axis response.41 Similarly, the combination of L. helveticus and L. rhamnosus prevented intestinal abnormalities and bacterial translocation to mesenteric lymph nodes, caused by chronic stress.42
Baily et al. demonstrated that prenatal stressors can alter the microbiome in rhesus monkeys by reducing the overall numbers of Bifidobacteria and Lactobacilli.52 Maternal separation model studies in rodents have shown that neonatal stress can significantly change the diversity and composition of gut microbiota, as well as the stress response.53,54 Moreover, in a mouse model exposed to chronic psychological stress, the gut microbial profile was altered, a decrease in Bacteroides and an increase in Clostridium genus was found, and pro-inflammatory cytokine IL-6 levels were increased.55 Humans under stress also show a modified microbial profile.56
Probiotic treatment has been able to reverse many of the stress related effects both in animals and humans. However, probiotic effect on corticosterone level seems to be strain specific.
L. helveticus and B. longum combined decreased stress-induced corticosterone secretion,40 whereas in another study Bifidobacteria were not found to affect the hormone levels.57 In a double blind, randomized parallel group study, healthy human subjects were given L. helveticus R0052 and B. longum in combination, or placebo for 30 days. The twenty-four hour urinary free cortisol (UFC) output was reduced in the group taking the probiotic combination.58
A complete understanding of the mechanisms underlying the interaction between gut microbiota and stress responses could provide with novel approaches to prevent and treat stress related defects with the use of psychobiotics.
Gut Microbiota and Anxiety
Multiple pathways mediate the effects of gut microbiota on the CNS, and many of these are implicated in the pathogenesis of mood disorders, including anxiety. Accumulating evidence indicates the role of gut microbiota in the pathophysiology of anxiety and other mood disorders,59,60 as well as the potential anxiolytic effects arising from its modulation (Table 2a, 2b).
Table 2a. Potential beneficial effects of different probiotic species on anxiety in mice.
Table 2b. Potential beneficial effects of different probiotic species on anxiety in humans.
Experiments conducted on animal models with altered gut microbial composition (GF, probiotics and/or antibiotics treated mice, enterically infected mice, fecal transplant), clearly showed a relation between gut microbiota and anxiety-related behavior. However, GF rodent models have given somewhat contradictory results. From one side, independent studies have demonstrated a reduced anxiety-like behavior in GF mice, as seen by increased exploration of generally aversive zones in the elevated plus maze (EPM), the light/dark test (L/D) and the open field (OF). Although GF mice have a reduced anxiety-like behavior, corticosterone plasma levels were significantly increased as compared to their SPF counterparts.15,16,65 On the other side, in one study female GF mice gave different results,66 and in another study GF rats showed both an increase in serum corticosterone and anxiety-like behavior.50 Reconstitution of gut microbiota in GF mice early in life, but not in adulthood, normalized some behavioral aspects.
Alterations in the monoamine systems have been associated with depression and anxiety. Interestingly, gut microbiota seems to have an impact on the serotonergic system. GF male mice showed increased levels of 5-HT and 5-hydroxyindoleacetic acid in the hippocampus,67 and L. helveticus treatment in hyperammonemia-treated rats led to an anxiolytic effect possibly through a reduction in hippocampal 5-HT levels.62
In a mouse model of induced anxiety and depression via olfactory bulbectomy, elevated CRH expression, increased c-Fos activity, 5-HT levels and colon motility were associated with an altered gut microbiome, suggested to be due to HPA axis activation.59 Rodent strains displaying anxiety symptoms, also showed an exaggerated HPA axis activation.68 A link between HPA axis dysfunction and anxiety is less clear than that with depression. However, evidence exists to support this notion, for instance in post-traumatic stress disorder.69
Furthermore, pathogen infection and GI inflammation in animal models has been shown to increase anxiety-like behavior. For example, in mice infected with Citrobacter rodentium anxiety-like behavior increased 7-8 hours post-infection, in the absence of a systemic immune response.70 In another study, infection with Trichuris muris in mice also led to increased anxiety-like behavior. Probiotic treatment with B. longum regularized infection-induced anxiety-like behavior, which was associated with decreased hippocampal brain-derived neurotrophic factor (BDNF) mRNA.71 Moreover, anxiety-like behavior associated with chronic colitis in mice, was attenuated by B. longum administration. The anxiolytic effect was vagally mediated, as it was not seen in vagotomized mice.63
Bifidobacterium and Lactobacillus genera are the main probiotics that show positive behavioral effects related to anxiety and depression.
In humans, anxiety and other mood disorders are often comorbid with medical conditions that are associated with an altered microbial composition, such as IBS and chronic fatigue syndrome (CFS). In a randomized, double-blind, placebo-controlled trial in CFS patients, administration of the probiotic L. casei Shirota decreased anxiety symptoms and increased the abundance of Bifidobacteria and Lactobacilli in the stool.64 In a clinical trial performed on healthy subjects, a probiotic combination of L. helveticus and B. longum showed a beneficial effect on anxiety and depressive measures, as well as a reduction in UFC, compared to the placebo group.61 Nevertheless, larger and meticulously designed clinical trials on patient population are needed to confirm probiotic health effects related to anxiety symptoms.
Gut Microbiota and Depression
Depression is a mood disorder of complex pathophysiology, causing significant disability and having severe social consequences.72 Important pathophysiological mechanisms involved in depression include neurotransmitter deficiencies, HPA axis dysfunction, reduced BDNF levels, increased proinflammatory cytokine levels and decreased neurogenesis.
As reported previously, it is evident that gut microbiota, through their link to the microbiota-gut-brain axis, can affect mood and behavior and thus the pathophysiology of depression,59 introducing the concept of psychobioma. Moreover, studies performed both in animals73 and humans, have demonstrated that depression is associated with an altered gut microbiota composition. A study performed on both depressed and healthy subjects, analyzed fecal microbiota and found a significant increase in the order Bacteroidales, specifically in Alistipes genus, and a decrease in Lachnospiraceae family in depressed individuals.74 Fecal samples of depressed and healthy individuals were analyzed in a similar study and showed that depressed patients had increased Bacteroidetes, Proteobacteria and Actinobacteria levels, and that Firmicutes levels were reduced. In more detail, they showed increased levels of Enterobacteriaceae and Alistipes, and reduced Fecalibacterium levels. This reduction negatively correlated with depressive symptoms severity.75
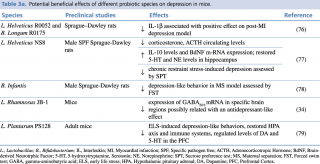
The use of probiotics has been extensively studied in animal models for both depression and anxiety symptoms (Table 3a, 3b).
Table 3a. Potential beneficial effects of different probiotic species on depression in mice.
Table 3b. Potential beneficial effects of different probiotic species on depression in humans.
Depression is frequently associated with HPA axis dysfunction, and normalization of the adverse effects resulting from this can contribute to the resolution of the disease. A hyperactivity of this axis is seen in GF mice, and its normalization is achieved with probiotics, such as B. infantis, suggesting antidepressant potential.
In a maternal separation model during the neonatal period in rats, chronic treatment with B. infantis reduced depressive-like symptoms, as seen by reduced immobility in the forced swim test (FST).78 The outcome was similar to that of citalopram. Probiotic treatment also normalized the immune response and NE concentrations in the brainstem. Administration of L. rhamnosus in healthy male BALB/c mice decreased anxiety- and depressive-like behavior in the EPM, OF and FST. The mice spent less time immobile in the FST. Probiotic treatment induced region-dependent alterations in gamma-aminobutyric acid (GABA) mRNA in a vagal-dependent manner.34 GABAB1b mRNA was increased in cortical cingulate and prelimbic regions, while it was decreased in the hippocampus, amygdala and locus coeruleus. Interestingly, in depressed animal models GABAB receptor expression was lower in frontal cortices.81 It was further observed that stress-induced elevation in corticosterone was reduced in L. rhamnosus fed mice. What is more, probiotic combination of L. helveticus and B. longum reversed the depressive behavior and restored intestinal barrier integrity in myocardial infarction (MI) rats. The study was performed in order to evaluate the preventive effect of probiotics on post-MI depressive behavior.76
Serotonin has an important role in the regulation of mood, and many antidepressants lead to serotonin increase as part of their mechanism of action. In the same study as mentioned previously, B. infantis resulted in increased levels of tryptophan, the precursor of serotonin, in the plasma of rats, thus indicating that probiotics can exert antidepressant effects by acting on the serotonergic system.78
Depression has been also associated with inflammation, seen by elevated levels in IL-6, tumor necrosis factor (TNF-α) and C reactive protein (CRP).82 It has been demonstrated that Lactobacillus and Bifidobacterium probiotics can stimulate anti-inflammatory IL-10 production in rodents, thus attenuating inflammatory responses.83 Additionally, low levels of BDNF is a hallmark of depression. Interestingly, gut microbiota seems to influence BDNF expression. A seminal study conducted in 200436 reported that GF male mice had a decrease in BDNF and expression of N-methyl-D-aspartate receptor subunit 2A in the hippocampus and the cortex, compared to controls. In another study, an increase in hippocampal BDNF mRNA65 was seen in female mice. Changes in the serotonergic system have also been observed in male but not female mice.67 Altogether there seems to be a sex dependent regulation of the microbiome-gut-brain axis. One possible antidepressant mechanism of gut microbiota is through the production of the SCFA butyrate. Sodium butyrate has been seen to produce an antidepressant effect in the murine brain, by affecting BDNF levels through its inhibitory action on histone deacetylase.84
An antidepressant effect was demonstrated by chronic administration of L. helveticus in a chronic-stress-induced depression model. Results showed that L. helveticus NS8 resulted in lower plasma corticosterone and ACTH levels, higher plasma IL-10 levels, normalized hippocampal 5-HT and NE levels, and increased hippocampal BDNF mRNA expression.77 Finally, L. plantarum PS128 was found to normalize HPA axis exaggerated response and depression-like behavior induced by early life stress (ELS). Moreover, chronic probiotic administration also repaired some ELS-induced immune and neurochemical changes.79 These studies clearly indicate a link between gut microbiota and the pathogenesis of depression.
Although less evidence exists for probiotic actions on human population, they seem to have similar anxiolytic and antidepressant effects as those seen in preclinical studies. For instance, a probiotic mixture of L. helveticus and B. longum, given to healthy subjects for 30 days, showed an amelioration in psychological distress compared to subjects on placebo. The assessment was performed by validated questionnaires and measurement of UFC.61 In a similar study, healthy subjects consumed a probiotic-containing milk drink of L. casei Shirota or placebo for 3 weeks. Mood was assessed both pre- and post-treatment, and subjects who scored in the lowest third for depressed mood showed a significant improvement at the end of treatment.85
In a randomized, double-blind, placebo-controlled clinical trial performed in patients with major depressive disorder, probiotics were administered to determine effects on depressive symptoms. The probiotic formulation contained L. acidophilus, L. casei and B. bifidum in a capsule. Patients who received the probiotic supplement had significantly decreased Beck Depression Inventory (BDI) scores.80
The “leaky gut” concept86 represents another link between gut and depression. Thus, probiotics able to reduce intestinal permeability could be considered useful in preventing depressive symptoms arising from intestinal dysfunction. However, there is a need for more clinical studies performed on affected population in order to make solid conclusions on the use of psychobiotics for depression.
Conclusions
Experimental research up to date has strongly demonstrated that gut microbiota is a key mediator of the bidirectional communication between the gut and the nervous system, thus leading to a paradigm shift in modern medicine and science. Moreover, gut microbiota has the ability to influence mood and behavior and it has been tightly associated with disease, ranging from gastrointestinal, through metabolic, to mental disorders, including anxiety and depression. Preclinical data has proven the role of gut microbiota in such disorders, as well as the use of probiotics as a therapeutic tool for improving related symptoms and as preventive means. However, caution must be exercised when translating preclinical results to clinical. Clinical research is still limited, but has provided with ambitious results regarding probiotic effects on neuropsychological disorders. Probiotics of the Bifidobacterium and Lactobacillus genera appear to have the most valuable benefits over anxiety- and depression-like behavior. It seems like gut microbiota is of crucial importance to a deeper understanding of the role the brain has in health and disease. Furthermore, modulation of the microbial composition with probiotics can offer new therapeutic and preventive approaches to combat mental diseases. Future clinical research focusing on the effects of psychobiotics on anxiety and depression in patient population will provide valuable information.
Acknowledgments: We thank Doctor Mario Bigioni for the figure and Doctor Paola Gualtieri for editing assistance.
Conflicts of Interest. No conflicts of interest, financial or otherwise are declared by the authors.
References
- Cryan JF, Dinan TG. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nature Reviews Neuroscience. 2012; 13(10):701-12.
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature. 2010; 464(7285):59-65.
- Xu J, Mahowald MA, Ley RE, Lozupone CA, Hamady M, Martens EC, et al. Evolution of Symbiotic Bacteria in the Distal Human Intestine. PLoS biology. 2007; 5(7):e156.
- Lay C, Sutren M, Rochet V, Saunier K, Dore J, Rigottier-Gois L. Design and Validation of 16S Rrna Probes to Enumerate Members of the Clostridium Leptum Subgroup in Human Faecal Microbiota. Environmental Microbiology. 2005; 7(7):933-46.
- Valles Y, Gosalbes MJ, de Vries LE, Abellan JJ, Francino MP. Metagenomics and Development of the Gut Microbiota in Infants. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012; 18 Suppl 4:21-6.
- Funkhouser LJ, Bordenstein SR. Mom Knows Best: The Universality of Maternal Microbial Transmission. PLoS biology. 2013; 11(8):e1001631.
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics. 2006; 118(2):511-21.
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human Gut Microbiome Viewed Across Age and Geography. Nature. 2012; 486(7402):222-7.
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the Human Gut Microbiome. Nature. 2011; 473(7346):174-80.
- Collins SM, Denou E, Verdu EF, Bercik P. The Putative Role of the Intestinal Microbiota in the Irritable Bowel Syndrome. Digestive and Liver Disease: Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2009; 41(12):850-3.
- Wang Y, Kasper LH. The Role of Microbiome in Central Nervous System Disorders. Brain, Behavior, and Immunity. 2014; 38:1-12.
- Sommer F, Backhed F. The Gut Microbiota - Masters of Host Development and Physiology. Nature Reviews Microbiology. 2013; 11(4):227-38.
- Kelly D, King T, Aminov R. Importance of Microbial Colonization of the Gut in Early Life to the Development of Immunity. Mutation Research. 2007; 622(1-2):58-69.
- Round JL, Mazmanian SK. The Gut Microbiota Shapes Intestinal Immune Responses During Health and Disease. Nature Reviews Immunology. 2009; 9(5):313-23.
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal Gut Microbiota Modulates Brain Development and Behavior. Proceedings of the National Academy of Sciences of the United States of America. 2011; 108(7):3047-52.
- Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of Intestinal Microbiota on Anxiety-Like Behavior. Communicative & Integrative Biology. 2011; 4(4):492-4.
- Tsigos C, Chrousos GP. Hypothalamic-Pituitary-Adrenal Axis, Neuroendocrine Factors and Stress. Journal of Psychosomatic Research. 2002; 53(4):865-71.
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: A Novel Class of Psychotropic. Biological Psychiatry. 2013; 74(10):720-6.
- Sherwin E, Rea K, Dinan TG, Cryan JF. A Gut (Microbiome) Feeling about the Brain. Current Opinion in Gastroenterology. 2016; 32(2):96-102.
- Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014; 34(46):15490-6.
- Tillisch K. The Effects of Gut Microbiota on CNS Function in Humans. Gut Microbes. 2014; 5(3):404-10.
- Douglas-Escobar M, Elliott E, Neu J. Effect of Intestinal Microbial Ecology on the Developing Brain. JAMA Pediatrics. 2013; 167(4):374-9.
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders with Suspected Immune Dysregulation. Clinical Therapeutics. 2015; 37(5):984-95.
- Al-Asmakh M, Anuar F, Zadjali F, Rafter J, Pettersson S. Gut Microbial Communities Modulating Brain Development and Function. Gut Microbes. 2012; 3(4):366-73.
- Mayer EA, Tillisch K, Gupta A. Gut/Brain Axis and the Microbiota. The Journal of Clinical Investigation. 2015; 125(3):926-38.
- Carabotti M, Scirocco A, Maselli MA, Severi C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Annals of Gastroenterology. 2015; 28(2):203-9.
- Bauer KC, Huus KE, Finlay BB. Microbes and the Mind: Emerging Hallmarks of the Gut Microbiota-Brain Axis. Cellular Microbiology. 2016; 18(5):632-44.
- Li Q, Zhou JM. The Microbiota-Gut-Brain Axis and Its Potential Therapeutic Role in Autism Spectrum Disorder. Neuroscience. 2016; 324:131-9.
- Mayer EA. Gut Feelings: The Emerging Biology of Gut-Brain Communication. Nature Reviews Neuroscience. 2011; 12(8):453-66.
- Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-Gut-Microbe Communication in Health and Disease. Frontiers in Physiology. 2011; 2:94.
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The Sympathetic Nerve - An Integrative Interface between Two Supersystems: The Brain and the Immune System. Pharmacological Reviews. 2000; 52(4):595-638.
- Cervi AL, Lukewich MK, Lomax AE. Neural Regulation of Gastrointestinal Inflammation: Role of the Sympathetic Nervous System. Autonomic Neuroscience: Basic & Clinical. 2014; 182:83-8.
- Forsythe P, Bienenstock J, Kunze WA. Vagal Pathways for Microbiome-Brain-Gut Axis Communication. Advances in Experimental Medicine and Biology. 2014; 817:115-33.
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proceedings of the National Academy of Sciences of the United States of America. 2011; 108(38):16050-5.
- D'Mello C, Ronaghan N, Zaheer R, Dicay M, Le T, MacNaughton WK, et al. Probiotics Improve Inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the Brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2015; 35(30):10821-30.
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. The Journal of Physiology. 2004; 558(Pt 1):263-75.
- Russell WR, Hoyles L, Flint HJ, Dumas ME. Colonic Bacterial Metabolites and Human Health. Current Opinion in Microbiology. 2013; 16(3):246-54.
- Lyte M. Probiotics Function Mechanistically As Delivery Vehicles for Neuroactive Compounds: Microbial Endocrinology in the Design and Use of Probiotics. Bioessays: News and Reviews in Molecular, Cellular and Developmental Biology. 2011; 33(8):574-81.
- Cryan JF, O'Mahony SM. The Microbiome-Gut-Brain Axis: from Bowel to Behavior. Neurogastroenterology and Motility: the Official Journal of the European Gastrointestinal Motility Society. 2011; 23(3):187-92.
- Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, et al. Probiotic Gut Effect Prevents the Chronic Psychological Stress-Induced Brain Activity Abnormality in Mice. Neurogastroenterology and Motility: the Official Journal of the European Gastrointestinal Motility Society. 2014; 26(4):510-20.
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, et al. Prevention of Gut Leakiness by a Probiotic Treatment Leads to Attenuated HPA Response to an Acute Psychological Stress in Rats. Psychoneuroendocrinology. 2012; 37(11):1885-95.
- Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, et al. Probiotics Prevent Bacterial Translocation and Improve Intestinal Barrier Function in Rats Following Chronic Psychological Stress. Gut. 2006; 55(11):1553-60.
- Chrousos GP, Gold PW. The Concepts of Stress and Stress System Disorders. Overview of Physical and Behavioral Homeostasis. Jama. 1992; 267(9):1244-52.
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-Adrenal and Autonomic Responses to Stress in Women after Sexual and Physical Abuse in Childhood. Jama. 2000; 284(5):592-7.
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005; 1:293-319.
- Cleck JN, Blendy JA. Making a Bad Thing Worse: Adverse Effects of Stress on Drug Addiction. The Journal of Clinical Investigation. 2008; 118(2):454-61.
- Watson S, Mackin P. HPA Axis Function in Mood Disorders. Psychiatry. 2006; 5(5):166-70.
- Hueston CM, Deak T. The Inflamed Axis: The Interaction between Stress, Hormones, and the Expression of Inflammatory-Related Genes within Key Structures Comprising the Hypothalamic-Pituitary-Adrenal Axis. Physiology & Behavior. 2014; 124:77-91.
- Smith SM, Vale WW. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues In Clinical Neuroscience. 2006; 8(4):383-95.
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Dauge V, et al. Absence of the Gut Microbiota Enhances Anxiety-Like Behavior and Neuroendocrine Response to Acute Stress in Rats. Psychoneuroendocrinology. 2014; 42:207-17.
- Gareau MG, Silva MA, Perdue MH. Pathophysiological Mechanisms of Stress-Induced Intestinal Damage. Current Molecular Medicine. 2008; 8(4):274-81.
- Bailey MT, Lubach GR, Coe CL. Prenatal Stress Alters Bacterial Colonization of the Gut in Infant Monkeys. Journal of Pediatric Gastroenterology and Nutrition. 2004; 38(4):414-21.
- O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, et al. Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biological Psychiatry. 2009; 65(3):263-7.
- Felice VD, Gibney SM, Gosselin RD, Dinan TG, O'Mahony SM, Cryan JF. Differential Activation of the Prefrontal Cortex and Amygdala Following Psychological Stress and Colorectal Distension in the Maternally Separated Rat. Neuroscience. 2014; 267:252-62.
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a Social Stressor Alters the Structure of the Intestinal Microbiota: Implications for Stressor-Induced Immunomodulation. Brain, Behavior, and Immunity. 2011; 25(3):397-407.
- Knowles SR, Nelson EA, Palombo EA. Investigating the Role of Perceived Stress on Bacterial Flora Activity and Salivary Cortisol Secretion: A Possible Mechanism Underlying Susceptibility to Illness. Biological Psychology. 2008; 77(2):132-7.
- Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria Exert Strain-Specific Effects on Stress-Related Behavior and Physiology in BALB/C Mice. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society. 2014; 2 6(11):1615-27.
- Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial Psychological Effects of a Probiotic Formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in Healthy Human Volunteers. Gut Microbes. 2011; 2(4):256-61.
- Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, et al. Altered Colonic Function and Microbiota Profile in a Mouse Model of Chronic Depression. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society. 2013; 25(9):733-e575.
- Bested AC, Logan AC, Selhub EM. Intestinal Microbiota, Probiotics and Mental Health: from Metchnikoff to Modern Advances: Part II - Contemporary Contextual Research. Gut Pathogens. 2013; 5(1):3.
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of Psychotropic-Like Properties of a Probiotic Formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in Rats and Human Subjects. The British Journal of Nutrition. 2011; 105(5):755-64.
- Luo J, Wang T, Liang S, Hu X, Li W, Jin F. Ingestion of Lactobacillus Strain Reduces Anxiety and Improves Cognitive Function in the Hyperammonemia Rat. Science China Life Sciences. 2014; 57(3):327-35.
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, et al. The Anxiolytic Effect of Bifidobacterium longum NCC3001 Involves Vagal Pathways for Gut-Brain Communication. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society. 2011; 23(12):1132-9.
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, et al. A Randomized, Double-Blind, Placebo-Controlled Pilot Study of a Probiotic in Emotional Symptoms of Chronic Fatigue Syndrome. Gut Pathogens. 2009; 1(1):6.
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced Anxiety-Like Behavior and Central Neurochemical Change in Germ-Free Mice. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society. 2011; 23(3):255-64, e119.
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, et al. Bacterial Infection Causes Stress-Induced Memory Dysfunction in Mice. Gut. 2011; 60(3):307-17.
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The Microbiome-Gut-Brain Axis During Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Molecular Psychiatry. 2013; 18(6):666-73.
- Jakovcevski M, Schachner M, Morellini F. Susceptibility to the Long-Term Anxiogenic Effects of an Acute Stressor Is Mediated by the Activation of the Glucocorticoid Receptors. Neuropharmacology. 2011; 61(8):1297-305.
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The Role of Corticotropin-Releasing Factor in Depression and Anxiety Disorders. The Journal of Endocrinology. 1999; 160(1):1-12.
- Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of Anxiety-Like Behavior in Mice during the Initial Stages of Infection with the Agent of Murine Colonic Hyperplasia Citrobacter Rodentium. Physiology & Behavior. 2006; 89(3):350-7.
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, et al. Chronic Gastrointestinal Inflammation Induces Anxiety-Like Behavior and Alters Central Nervous System Biochemistry in Mice. Gastroenterology. 2010; 139(6):2102-12.e1.
- Association AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013.
- De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, et al. Microbiota and Host Determinants of Behavioural Phenotype in Maternally Separated Mice. Nature Communications. 2015; 6:7735.
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, et al. Correlation between the Human Fecal Microbiota and Depression. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society. 2014; 26(8):1155-62.
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain, Behavior, and Immunity. 2015; 48:186-94.
- Arseneault-Breard J, Rondeau I, Gilbert K, Girard SA, Tompkins TA, Godbout R, et al. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 Reduces Post-Myocardial Infarction Depression Symptoms and Restores Intestinal Permeability in a Rat Model. The British Journal of Nutrition. 2012; 107(12):1793-9.
- Liang S, Wang T, Hu X, Luo J, Li W, Wu X, et al. Administration of Lactobacillus helveticus NS8 Improves Behavioral, Cognitive, and Biochemical Aberrations Caused by Chronic Restraint Stress. Neuroscience. 2015; 310:561-77.
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the Probiotic Bifidobacterium Infantis in the Maternal Separation Model of Depression. Neuroscience. 2010; 170(4):1179-88.
- Liu YW, Liu WH, Wu CC, Juan YC, Wu YC, Tsai HP, et al. Psychotropic Effects of Lactobacillus Plantarum PS128 in Early Life-Stressed and Naive Adult Mice. Brain Research. 2016; 1631:1-12.
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, et al. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition (Burbank, Los Angeles County, Calif). 2016; 32(3):315-20.
- Cryan JF, Kaupmann K. Don't Worry 'B' Happy!: A Role For GABA(B) Receptors in Anxiety and Depression. Trends in Pharmacological Sciences. 2005; 26(1):36-43.
- Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, et al. Major Depression is Associated with Significant Diurnal Elevations in Plasma Interleukin-6 Levels, a Shift of Its Circadian Rhythm, and Loss of Physiological Complexity in Its Secretion: Clinical Implications. The Journal of Clinical Endocrinology and Metabolism. 2005; 90(5):2522-30.
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The Probiotic Bifidobacteria Infantis: An Assessment of Potential Antidepressant Properties in the Rat. Journal of Psychiatric Research. 2008; 43(2):164-74.
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-Like Effects of the Histone Deacetylase Inhibitor, Sodium Butyrate, in the Mouse. Biological Psychiatry. 2007; 62(1):55-64.
- Benton D, Williams C, Brown A. Impact of Consuming a Milk Drink Containing a Probiotic on Mood and Cognition. European Journal of Clinical Nutrition. 2007; 61(3):355-61.
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking Down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-Related Psychiatric Disorders. Frontiers in Cellular Neuroscience. 2015; 9:392.