Zika virus: the fear travels by mosquitoes - Social and psychological impact of the outbreak
Orlando Cenciarelli,1,2*† Mariachiara Carestia,1,2 Stefano Pietropaoli,3 Gian Marco Ludovici,2 Valentina Gabbarini,2 Sandro Mancinelli,2,4 Andrea Malizia,1,2* Daniele Di Giovanni,1,2 Pasquale Gaudio1,2 and Leonardo Palombi2,4
1Department of Industrial Engineering, University of Rome Tor Vergata, Rome, Italy;
2International Master Courses in Protection Against CBRNe events, Department of Industrial Engineering and School of Medicine and Surgery, University of Rome Tor Vergata, Rome, Italy;
3Department of Science, University of Rome 3, Rome, Italy;
4Department of Biomedicine & Prevention, School of Medicine and Surgery, University of Rome Tor Vergata, Rome, Italy.
ZIKV Background
Zika virus (ZIKV) is an emerging mosquito-borne arbovirus belonging to the genus Flavivirus, family of Flaviviridae, firstly isolated in April 1947 from a Macaca mulatta (also known as Rhesus monkey) caged in the Zika Forest (Uganda).1 The human transmission occurs mainly through the bite of an infected mosquito belonging to the genus Aedes,2 the same mosquitoes that may transmit other viruses such as Dengue virus (DENV), Chikungunya virus (CHIKV) and Yellow Fever virus (YFV);3 however other transmission routes (perinatal, in utero, and possible sexual and by blood transfusion) are reported.2 Human and non-human primates represent the main virus reservoirs;2 usually the clinical manifestations are similar, but often mildest, with respect to other mosquito-borne virus, as DENV or CHIKV.4,5
Molecular Features
The genome of ZIKV is a positive-sense single-stranded RNA, containing 10,794 nucleotides including 2 flanking non-coding regions (5’ and 3’ NCR). The single Open Reading Frame (ORF) encodes for three structural proteins, E (envelope glycoproteins), C (capsid protein) and M (membrane protein)6 and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5), which carry out crucial functions in assembly and replication. E protein (53 kDa) is the virion surface protein, it is involved in several aspects of the viral cycle, mediating binding and membrane fusion. The host response to the pathogen is probably mediated by one or more of the NS proteins;7while the 5’ and 3’ untranslated regions have proved necessary to the cyclization and replication of the virus.7 Furthermore, these sequences appear to be strongly linked to the same regions of other related Flaviviruses. Genomic studies have identified more subclasses of ZIKV, pointing to three major lineages: one Asian and two African.8
Transmission Routes
ZIKV is transmitted by arthropods (mosquitoes genus Aedes) as it happens for YFV, West Nile virus (WNV), Japanese encephalitic virus (JEV) and DENV.5 ZIKV has been isolated from A. aegypti, A. africanus, A. luteocephalus and A. apicoargenteus;9 A. hensilii has been the principal vector during the ZIKV outbreak in Yap State (Micronesia) in 2007.6 These mosquitoes species lay the eggs in pots, buckets and mangers, near to human settlements; this makes mosquitoes particularly aggressive and able to feed also during the day.2 ZIKV is transmitted from the vector to reservoir animals through a bite;5 if non-infected mosquitoes bite infected people, they can acquire and further spread the virus.10 Maternal-fetal transmission of ZIKV has been confirmed in pregnant women;11 conversely, the transmission by blood transfusion and sexual relations are not yet fully confirmed.4,12
Pathogenesis
The symptoms appear after 3-12 days from the bite of an infected mosquito.13 Generally, travelers from affected areas, show the symptoms up to six days since they left the endemic areas.4 Studies on the sexual transmission of ZIKV evidence that symptoms appear approximately 10 days after sexual intercourse.4 Approximately 80% of people infected by ZIKV are asymptomatic,2 the remaining 20% can show a broad range of clinical symptoms,14 similar to those caused by DENV or CHIKV. Symptoms may include fever, headache, cough, sore throat, arthralgia, myalgia, conjunctival hyperemia, vertigo, edema at the extremities of the body, maculopapular rash and gastrointestinal disorders like vomiting. Usually the symptoms disappear spontaneously after 3-7 days, but some evidences suggest that the arthralgia can persist for about a month.14
Recently, the presence of ZIKV has been observed in people affected by Guillain-Barré syndrome (GBS), suggesting the hypothesis that there may be a correlation between the GBS and the ZIKV infection.15 Moreover, pregnant women infected by ZIKV have given birth to children affected by microcephaly; this correlation has been documented in Brazil after a significant increase in case of newborn babies with microcephaly in the regions involved in the active ZIKV transmission.16 However, even if it should be emphasized that there is currently no definite correlation between the virus and the disease, because the microcephaly depends on multiple factors,11 the correlation between ZIKV infection and microcephaly has generated globally serious concerns.
Diagnosis
ZIKV infection is quite difficult to detect for several reasons; first of all it is often confused with DENV or CHIKV infections. DENV, CHIKV and ZIKV share similar symptoms, vector and distribution areas.17 While fast tests to verify infection by DENV or CHIKV are commercially available, currently there is no approved test for ZIKV identification.2 The most reliable way to verify the presence of ZIKV is based on the detection of viral RNA in blood samples through RT-PCR;2 however, as a consequence of the short viremic period, blood sample must be collected before the fifth day from the onset of symptoms.18 After that period, it is possible to use the same technique to identify the virus in the urines: viruria is believed to last longer than viremia.18 Other techniques for ZIKV diagnosis are the pan flavivirus amplification technique combined with sequencing6 and the less reliable serological test to detect anti-Zikv IgM and IgG.2 The main trouble related to serological tests, as ELISA or immunofluorescence techniques, is the cross-reaction with other flavivirus, like DENV, that can make the diagnosis challenging.19 Moreover, IgM and IgG levels can be quite low in the first phase of the disease.6 CDC recommend, anyhow, the use of both RT-PCR and serological approaches to verify possible ZIKV infections.2
Progressive Geographical Distribution
Discovered in Uganda in 1947, the first human case of ZIKV infection was identified in Nigeria in 1954.4 ZIKV remained almost unknown to common people until last month, when the media began to spread the news of several cases in South America. While, since few months ZIKV is affecting a number of different continents, only few cases occurred in the past (Table I, Figure 1). Since 1951 to 1981, human ZIKV infection cases were confirmed in Nigeria, Uganda, Ivory Coast, Burkina Faso, Central African Republic, Senegal, Gabon, Cameroon, Cambodia, Malaysia, the Philippines, Thailand, and Indonesia,6,20consisting in two distinct virus strains in Africa and Asia.14 Since then, a small number of other cases was reported till 2007, when 108 cases of ZIKV infection were confirmed from April to August in Yap, Federated States of Micronesia.5 In October 2013, a large outbreak occurred in French Polynesia.5 The same year, the first case of ZIKV infection was imported in Canada from Thailand.21 From 2014, the distribution of ZIKV infection cases is progressively raising concern globally (Table II, Figure 2). Starting from the two infected Italian tourists returning from the French Polynesia,4 many cases had been notified to the Pan American Health Organization/World Health Organization (PAHO/WHO).22,23In February 2014, first ZIKV infection was reported in Chile, where confirmed cases were recorded until summer.23 During the last 9 months, the threat is gradually increasing. In May 2015, the confirmation of ZIKV infection in the northeast of Brazil arouse the alert in South America,2 where just five months later 14 states confirmed local virus spread (Alagoas, Bahia, Ceará, Maranhão, Mato Grosso, Pará, Paraná, Paraíba, Pernambuco, Piauí, Rio de Janeiro, Rio Grande do Norte, Roraima, and São Paulo).22,23 Also Colombia health authorities reported the detection of the first local case of ZIKV infection in the state of Bolívar.24 In November, the spread reached Mexico (2 local cases and one acquired from Colombia), El Salvador, Guatemala, Paraguay, Venezuela, Suriname,22 Samoa and American Samoa. Since then, ongoing virus transmission has been reported also in Costa Rica and Nicaragua.22,24 At the beginning of December, 3 cases had been laboratory-confirmed in Panama.22 Since then, ZIKV infection cases had been notified in Cape Verde, Honduras,22 and others in Panama.22 In December, four different cases of ZIKV infection had been notified in two overseas regions of France: French Guiana and Martinique.22 At the end of the year, a first case was recorded in the Commonwealth of Puertorico.22 On January 2016, the fear towards ZIKV came to the fore once again. From that moment, the Caribbean Island of Curaçao has reported ongoing transmission of ZIKV.24 Between 4 and 12 January, two cases of acquired infection were recorded in Germany, in travelers returning from Haiti at the end of the year.22 On 14 January, a case of locally-acquired infection was recorded in Guyana. The day after, three patients resulted ZIKV-positive in Barbados, in the Lesser Antilles, and two in Ecuador. Since 16 January 2016, two locally-acquired cases and four imported cases (3 from Colombia and 1 from Venezuela) were reported.22 On 16 January, even Bolivia seemed to be no ZIKV-free: a pregnant woman from Portachuelo resulted positive to the infection, without any recent travel history.22 As a confirmation of the acquired nature of the ZIKV infection in the German travelers, on 18 January, five Haitian patients resulted positive to the virus.22 In the same time, two other cases were remarked in France, in the overseas departments of Saint Martin and Guadeloupe.22 On 23 January, 10 laboratory-confirmed cases, of which 8 locally-acquired and 2 imported from El Salvador, were notified in the Dominican Republic.22 On 25 January in one of the United States Virgin Islands (USVI) the first case of ZIKV infection was notified.22 On 16 January an imported case from Maldives of ZIKV was notified in Finland. The case dates back to June 2015, and was the only case identified in Maldives.22 On 30 January the first case of ZIKV infection was notified in Jamaica;22 and on 3 February the first case of ZIKV infection was reported in Tonga, a Polynesian archipelago.24 On 5 February the first probable case of sexual transmission of ZIKV was notified in USA in a person that had sex with a ZIKV affected patience returned from a trip in Venezuela, there the infection was active.22 On 15 and 16 February, first cases of autochthonous transmission of ZIKV were reported in two islands part of the Netherlands situated in the southern part of the Carribean region, Bonaire and Aruba, respectively. On 18 February, the first case of autochthonous transmission of ZIKV was reported in Trinidad and Tobago;22 few days later, on 23 February, first case of ZIKV local transmission was reported in Marshall Islands.24 On 25 February the first cases of ZIKV were reported in Saint Vincent and Grandines and in Sint-Marteen, isles part of the Lesser Antilles and the Netherlands Antilles.22 On 29 February, a potential sexual transmitted case of ZIKV infections was reported in the province of Cordoba, Argentina, in a person that had unprotected sex with an imported case who travelled to Colombia, a ZIKV affected country.22 On 1 March, a potential case of sexual transmission of ZIKV was reported in France, in a person that had unprotected sex with an imported case who travelled to Brazil, a ZIKV affected country.22 On 9 March another Pacific Island, the French territory of New Caledonia reports the first case of active transmission of ZIKV infection.24 On 11 March, the results of a retrospective testing of samples taken from July 2014 to March 2016 from person showing ZIKV-like symptoms were notified by Papua New Guinea. Three cases were confirmed from samples collected in 2015 and three cases from samples collected in 2016.22 On 15 March, the first autochthonous case of ZIKV was reported in Dominica; the following day the first case was reported in Cuba.22 On 26 March a putative case of sexual transmission of ZIKV was reported in Chile in a person that had sex with an affected patience travelled to a country where ZIKV transmission was active.22 On 1 April the first case of ZIKV active transmission was reported in Kosrae, an island in Federated States of Micronesia;24 the following day, 2 April, two cases were notified in Vietnam, resulting from the screening of 1215 samples collected from people with ZIKV-like symptoms.22 On 4 April another island country in the South Pacific Ocean, the Fiji, reported active ZIKV transmission;24 on 7 April, two autochthonous cases of ZIKV infection were reported in Saint Lucia island.22 On 17 April, the first case of sexual transmission of ZIKV was reported in Perù in a person after sexual contact with a patient who travelled in a ZIKV affected country.22Finally, on 18 April the active transmission of ZIKV infection was reported in Belize, a country on the eastern coast of Central America.
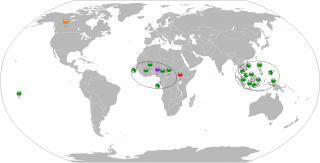
Table I. Countries involved in ZIKV active transmission or imported cases. Period 1954-2013. The diffusion in 1954 represents the first human diffusion after the identification of the virus in Uganda in 1947.
Figure 1. Geographical global vision of countries involved in ZIKV active transmission or imported cases. Period 1954-2013. Red map pin indicates the first identification of ZIKV in non-human primate, Uganda 1947. Purple map pin indicates the first human case of ZIKV in Nigeria in 1954. Green map pins indicate the human cases in the period 1954-2013 except the first one. The orange map pin indicates the imported case in Canada, 2013. Black circles virtually collect the human cases in an African and an Asian zone.
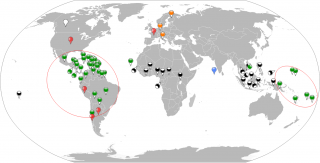
Table II. Countries involved in ZIKV active transmission or imported cases. Period 2014- 1st May 2016. Several Countries of South America and Pacific Islands are currently experiencing a large outbreak of ZIKV without precedent since the virus was discovered.
Figure 2. Geographical global vision of countries involved in ZIKV active transmission (by vector or by sexual route) or imported cases. Period 2014 - 1st May 2016. Green map pins indicate the human cases in the period 2014- 1st May 2016. The orange map pins indicate the imported cases in Italy, Germany and Finland. The black and white map pins indicate the human cases in the period 1954-2014. The red map pins indicate the sexual transmitted cases. The blue map pin indicates the only local exported case in Maldives. Red circles virtually collects the human cases in South American and Pacific Islands zones.
Correlation between ZIKV and Microcephaly
A twenty fold, alarming, increase in diagnosis of fetal and pediatric microcephaly has been reported in Brazil during the last year. By 29 November 2015, 646 cases have been reported in Prnambuco state alone and Brazilian authorities are considering the association with the outbreak of ZIKV disease. The increase was so alarming that the Brazil Ministry of Health promptly developed a case definition for ZIKV-related microcephaly, and a task force and registry were established to investigate and to describe the clinical characteristics of cases.25 Experimental evidences showed that ZIKV infection is associated with autophagy in skin fibroblasts, and its involvement in the same process in neural cells cannot be ruled out. This is an indication of the association of ZIKV to fetal microcephaly, in association with the observation of centrosomes abnormalities (closely linked to microcephaly) resulting from the infection. Together with the apparently inexplicable high prevalence of microcephaly occurred in Brazil in 2015, these clues depose in favor of the association of this infection to fetal microcephaly. Nonetheless, evidences to bring a charge against ZIKV are still required.26 Interestingly, literature reports about the cases of two pregnant women diagnosed with fetal microcephaly that had negative blood results for ZIKV, but positive RT-PCR results for amniocentesis, supporting the evidences of intrauterine transmission of the virus and its likely activity after the viremic period in the pregnant women. Ultrasound images of microcephaly diagnosed fetus reveal damages that can be correlated to those reported for WNV fetal encephalitis, rather than abnormalities typical of other intrauterine infections that affect the brain (e.g. toxoplasmosis, syphilis, rubella), further supporting the assumption of a direct link between ZIKV intrauterine infection and microcephaly.27
Virus Spread and Limits to the Containment
ZIKV has quickly become a public health crisis. Numerous factors can be considered among the contributing causes of this explosive spread; the causes can be looking in several factors, mainly dependent on human activities. One of the negative aspects of globalization is undoubtedly the opportunity for pathogens to reach unnatural places respect the original areas. Among zoonoses, the vector-borne diseases are the pathogens most affected by globalization in terms of their spread; changes in lifestyle and the reduction of geographic barriers makes very uncertain the boundaries between different areas.28 During the last 20 years, the movement of human masses has been increasing exponentially.29 Thanks to the continuous development of low cost fast travels, a growing number of people is able to reach distant countries in less than a day. To the number of people traveling for business or pleasure, millions of people per year, move for humanitarian reasons, fleeing from war, persecution, and poverty. All these factors, can promote the spread of the outbreaks of infectious diseases, previously confined at the local level.29 In this scenario, the capability of ZIKV vector to adapt to several transportation means, like goods shipping,30 and the possibility that an infected person can, in few hours, reach several distant countries facilitate the virus spread.31 Before the burst of ZIKV infections, recent outbreaks of DENV (2012) and CHIKV (2007) in Southern Europe evidenced the ease of emergence of new outbreaks from infected travelers. In both cases, the virus transmission was due to a passenger that visited affected countries. In 2012, the province of Madeira, Portugal, reported a DENV outbreak, with 2168 affected people. More than fifty patients returning from Madeira were diagnosed in other European countries with DENV infection.32 In 2007, first CHIKV outbreak in Italy was recorded, with over 200 affected individuals; the infection started from an infected traveler returning from India.33 The spread of vectors, their ability to become invasive,34 and the increase of travels, to and from affected countries, seriously increase the risk for new outbreaks of tropical infective diseases such as ZIKV in non-endemic areas.29 On the other hand, a crucial role in the spread of vector-borne diseases is played by climate change, the global temperature increase on top. Correlations between the temperature increase and outbreaks of DENV are reported by the literature;35 since ZIVK shares the vector of diffusion with DENV, it is easy to relate an enhanced spread of ZIKV with climate change. The rise in temperature and global climate change allows a progressive movement of the ZIKV vector, A. aegipti, outside the traditional areas of diffusion; consequently, the distribution area of the virus moves together with the vector. Moreover, the temperature rise has an inverse proportionality with the time necessary for the development of mosquito larvae. In particular, 2015, has proven to be the year with the largest rise in global temperature; extending the mosquito season, and resulting in more time for vectors to bite and infect humans. In addition, increasingly heavy rain seasons resulting in the formation of puddles (especially in South America, where an exceptional El Niño caused an intense rains season) created ideal habitats for breeding of mosquitoes.36 The reproduction of mosquitoes, is also enhanced in urban environments inhabited by poor communities, in which, the lack of running water, lead the population to use bins or other open containers for water domestic stockpiling. Rising temperatures, finally, has an indirect effect on the virus spread, since clothes represent a physical barrier that protects people from mosquito bites; higher temperatures pushes people to wear short sleeve cloths increasing the areas of the body exposed to the bite of mosquitoes.37 All these factors limit the management of the spread of the virus. Currently, several strategies for the containment of the vector spread, in particular in urban environments are put into practice to limit the spread of A. aegipti, especially by insecticide administration. However, it is easy intuitive how unbalanced is this struggle; in fact it is not possible to completely decontaminate the breeding and life grounds of mosquitoes. Also restricting the movements of people and goods, and consequently the spread of the vector in far areas respect to the distribution areal of the virus, is extremely challenging.
Social and Psychological Impact of ZIKV Diffusion
If on one hand, ZIKV disease symptoms for affected adult population are modest and should be a moderate concern for public healthcare settings and governmental institutions, on the other, the possible effects of ZIKV on pregnant woman would result in a high impact.
The psychosocial implication related to the ZIKV outbreak may be more dangerous than any potential acute physical effect.
In fact, the onset of psychogenic illnesses has often been reported following events such the WNV outbreaks in the US,38 a virus that is closely linked to ZIKV. Moreover, when the threat is represented by an emerging disease, the lack of medical and scientific knowledge, as well as of means of prophylaxis or specific treatments may blow panic out of proportions. Given the mild effects of the ZIKV disease, in this specific case, the most likely source of psychosis and social disruption would be related to the possible correlation of the disease with fetus microcephaly. Pregnant women and their relatives would consequently be a highly sensitive risk group. The psychosis generated by this kind of outbreaks may have consequences in terms of healthcare settings overload and mistrust towards national authorities.
At this regard, public psycho-educational support and an effective communication strategy can reduce or limit adverse population reactions, promoting self-protecting behaviors and reducing the spread of panic as demonstrated by a number of initiatives promoted, for example, in the aftermath of the 2001 World Trade Center disaster,39 and during the WNV outbreak in New York in 1999.
Conclusions
The WHO has declared ZIKV infection an international health emergency; ZIKV is spreading at an alarming rate and the prediction of its diffusion over time will be extremely challenging.17,22
Despite its low lethality, the ZIKV spread could result in massive psychological consequences due to the growing evidences between the disease and fetus microcephaly.40 Moreover, the cases that have been reported so far, concerning tourists infected by the virus and traveling back to their countries contribute to the increase of uncertainties on the spread of the disease and the subsequent general insecurity and fears. Moreover, climate changes as well as the massive movement of people and goods across the globe stand in the way of an effective strategy for the outbreak confinement.
To boost resilience and preparedness and to reduce the impact of this kind of scenario, but also to mitigate the consequences of a natural outbreak a set of measures should be considered. On the one side the physical spread of the disease must be contained through an aggressive control of Aedes species that can become vectors of the virus and the provision of facilities for a fast and accurate diagnosis of ZIKV disease promptly available in all the critical areas). On the other side, the psycho-social repercussion of the possible outbreaks needs to be contained by implementing effective emergency communication and support strategies.
Addendum
Due to the rapid evolution of the Zika virus outbreak, all the data shown in figures and/or table of this paper represents the situation on May 1st, 2016.
Acknowledgments
Special acknowledgement for the realization of this work goes to the International Master Courses in “Protection Against CBRNe Events” (http://www.mastercbrn.com).
Acknowledgements
Special acknowledgements for the realization of this work go to the International Master Courses in “Protection Against CBRNe Events” (http://www.mastercbrn.com).
References
-
Dick GWA, Kitchen SF, Haddow AJ. Zika virus (I). Isolations and Serological specificity. T Roy Soc Trop Med H. 1952; 46:509-20.
-
Centers for Disease Control and Prevention. Zika Virus. [cited 2016 May 1]. http://www.cdc.gov/zika/
-
World Health Organization. Zika Virus [cited 2016 May 1]. http://www.who.int/mediacentre/factsheets/zika/en/
-
Zammarchi L, Tappe D, Fortuna C, Remoli ME, Günther S, Venturi G, et al. Zika Virus Infection in a Traveller Returning to Europe from Brazil, March 2015. Euro Surveill. 2015;20:14-16.
-
Ioos S, Mallet HP, Goffart IL, Gauthier V, Cardoso T, Herida M. Current Zika Virus Epidemiology and Recent Epidemics. Med Maladies Infect. 2014; 44:302-7.
-
Hayes EB. Zika Virus outside Africa. Emerg Infect Dis. 2009; 15:1347-50.
-
Gatherer D, Kohl A. Zika Virus: A Previously Slow Pandemic Spreads Rapidly through the Americas. J Gen Virol. 2015.
-
Faye O, Freire CC, Iamarino A, Faye O, de Oliveira, JVC, Diallo M, et al. Molecular Evolution of Zika Virus during Its Emergence in the 20th Century. PLoS Negl Trop Dis, 2014; 8(1):e2636.
-
McCrae AWR, Kirya BG. Yellow Fever and Zika Virus Epizootics and Enzootics in Uganda. T Roy Soc Trop Med H. 1982; 76:552-62.
-
Higgs S, Vanlandingham D. Chikungunya Virus and its Mosquito Vectors. Vector Borne Zoonotic Dis. 2015; 15:231-40.
-
Petersen, E. Interim Guidelines for Pregnant Women during a Zika Virus Outbreak – United States, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65:30-33.
-
Musso D, Nilles EJ, Cao Lormeau VM. Rapid Spread of Emerging Zika Virus in the Pacific Area. Clin Microbiol Infec. 2014; 20:595-96.
-
Hennessey M. (2016). Zika Virus Spreads to New Areas – Region of the Americas, May 2015–January 2016. MMWR Morb Mortal Wkly Rep. 2016; 65:55-58.
-
Kwong JC, Druce JD, Leder K. Zika Virus Infection Acquired during Brief Travel to Indonesia. Am J Trop Med Hyg. 2013; 89:516-17.
-
Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, et al. Zika Virus Infection Complicated by Guillain-Barré Syndrome-case Report, French Polynesia, December 2013. Euro Surveill. 2014; 19:20720.
-
The Lancet Editorial. Zika Virus: A New Global Threat for 2016. Lancet 2016:387:96.
-
Musso D, Cao-Lormeau VM, Gubler DJ. Zika Virus: Following the Path of Dengue and Chikungunya. Lancet. 2015; 386:243-44.
-
Kutsuna S, Kato Y, Takasaki T, Moi ML, Kotaki A, Uemura H, et al. Two Cases of Zika Fever Imported from French Polynesia to Japan, December 2013 to January 2014. Euro Surveill. 2014; 19:1-5.
-
Tappe D, Rissland J, Gabriel M, Emmerich P, Günther S, Held G, et al. First Case of Laboratory-confirmed Zika Virus Infection Imported into Europe, November 2013. Euro Surveill. 2013; 54:54.
-
Fauci AS, Morens DM. Zika Virus in the Americas – Yet Another Arbovirus Threat. N Engl J Med. 2016. In press.
-
Fonseca K, Meatherall B, Zarra D, Drebot M, MacDonald J, Pabbaraju K, et al. First Case of Zika Virus Infection in a Returning Canadian Traveler. Am J Trop Med Hyg. 2014; 91:1035-38.
-
World Health Organization. Emergencies Preparedness, Response. Disease Outbreak News (DONs) [cited 2016 May 1]. http://www.who.int/csr/don/en/
-
Pan American Health Organization. Epidemiological Update. Zika Virus Infection [cited 2016 Jan 31]. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32021&lang=en
-
Centers for Disease Control and Prevention. Zika Travel Information [cited 2016 May 1]. http://wwwnc.cdc.gov/travel/page/zika-travel-information
-
Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DDG, Cavalcanti DP, Pessoa A, et al. Possible Association between Zika Virus Infection and Microcephaly – Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016; 65:59–62.
-
Tetro JA. Zika and Microcephaly: Causation, Correlation, or Coincidence? Microbes and Infection. In press 2016.
-
Oliveira Melo AS., Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika Virus Intrauterine Infection Causes Fetal Brain Abnormality and Microcephaly: Tip of the Iceberg? Ultrasound Obst Gyn. 2016; 47:6-7.
-
Failloux AB, Moutailler, S. Zoonotic Aspects of Vector-borne Infections. Revue scientifique et technique (International Office of Epizootics), 2015; 34:175-83.
-
World Health Organization. International Travel and Health: Situation as on 1 January 2010. [cited 2016 Jan 31]. http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=18&codcch=10#
-
Moore CG, Mitchell CJ. Aedes Albopictus in the United States: Ten-year Presence and Public Health Implications. Emerg Infec Dis. 1997; 3:329.
-
Assistance Publique Hopitaux de Marseille. Maladies Infectieuses et Tropicales (MIT) – Hôpital Nord. [cited 2016 Jan 31]. http://fr.ap-hm.fr/service/mit-maladies-infectieuses-et-tropicales-hopital-nord
-
Sousa CA, Clairouin M, Seixas G, Viveiros B, Novo MT, Silva AC, et al. Ongoing Outbreak of Dengue Type 1 in the Autonomous Region of Madeira, Portugal: Preliminary Report. Euro Surveill. 2012; 17:20333.
-
Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. Infection with Chikungunya Virus in Italy: An Outbreak in a Temperate Region. Lancet. 2007; 370:1840-6.
-
Straetemans M, ECDC Consultation Group on Vector-Related Risk for Chikungunya Virus Transmission in Europe. Vector-related Risk Mapping of the Introduction and Establishment of Aedes Albopictus in Europe. Euro Surveill. 2008; 13.
-
Mohd-Zaki AH, Brett J, Ismail E, L’Azou M. Epidemiology of Dengue Disease in Malaysia (2000–2012): A Systematic Literature Review. PLoS Negl Trop Dis. 2014; 8:e3159.
-
Patz JA, Githeko AK, McCarty JP, Hussein S, Confalonieri U, De Wet N. Climate Change and Infectious Diseases. Climate Change and Human Health: Risks and Responses. 2003; 6:103-37.
-
Danforth ME, Reisen WK, Barker CM. The Impact of Cycling Temperature on the Transmission of West Nile Virus. J Med Entomol. 2016; tjw013.
-
Covello VT, Peters RG, Wojtecki JG, Hyde RC. Risk Communication, the West Nile Virus Epidemic, and Bioterrorism: Responding to the Communication Challenges Posed by the Intentional or Unintentional Release of a Pathogen in an Urban Setting. J Urban Health. 2001; 78:382-391.
-
Boscarino JA, Adams RE. Assessing Community Reactions to Ebola Virus Disease and Other Disasters: Using Social Psychological Research to Enhance Public Health and Disaster Communications. Int J Emerg Ment Health. 2015; 17:234.
-
Brasil P, Pereira JP, Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro – Preliminary Report. N Engl J Med. 2016; DOI: 10.1056/NEJMoa1602412