Metals dyshomeostasis in Alzheimer’s Disease: A Systematic Review.
Lucaroni F,1* Ambrosone C,1 Paradiso F,1 Messinese M,1 Di Domenicantonio R,2 Alessandroni C,1 Cerone G,1 Cerutti F,1 Di Gaspare F,1 Morciano L,1 Tran L,3 Pietroiusti A,1 Palombi L.1
1 Department of Biomedicine and Prevention, University of Rome Tor Vergata – Rome, Italy
2 School of General Practice – Lazio Region, Italy
3 Institute of Occupational Medicine – Edinburgh, United Kingdom
Introduction
Dementia represents a major healthcare problem, involving 46.8 million people worldwide, with over 9.9 million new cases every year, one every 3.2 seconds.1 Dementia is a leading cause of disability and dependency in the elderly, leading to high physical, psychological, social and economic impact on caregivers and families. It is also responsible for higher direct and indirect costs to society. The estimated worldwide cost of dementia is US $818 billion and is expected to reach one trillion dollars by 2018.1
Alzheimer’s disease (AD) is the most frequent cause of dementia, involving 50 to 75 per cent of the global burden of disease,2 with almost 46 billion people affected worldwide3 and 1.54 million deaths globally.4 Indeed, according to WHO,4 Alzheimer’s disease is the seventh cause of death among high-income countries. Therefore, preventing AD is a main issue: unfortunately, the detailed knowledge of its pathogenesis that would be necessary in order to implement rational preventive measures, is not yet available.
AD is a neurodegenerative disorder, characterized by progressive brain deposition of the amyloid β peptide (Aβ), which is generated by proteolytic cleavage of amyloid precursor protein (APP) by β- and γ-secretases. The abnormal aggregation and accumulation of neurotoxic Aβ has been proposed as the primary driving force for AD in the amyloid hypothesis.5
Amyloid-β is also a major constituent of senile plaques. According to recent studies, the cerebral accumulation of metal ions might contribute to Aβ aggregation, through the production of a reactive oxygen species (ROS).6 Although there is a plethora of studies concerning pathogenesis of AD, it has not been completely clarified yet because of the complex interaction between endogenous (age, family history, genetic factors, gender) and exogenous (environment) factors that could contribute to the development of this illness.7 Genetic predisposition seems to be the most important risk factor of Alzheimer’s disease, contributing 70% of the overall risk of AD, with the remaining 30% likely to be caused by lifestyle and chronic exposure to environmental factors.8 The “brain trace metal dyshomeostasis” is a fascinating, although controversial, hypothesis to explain the residual part of ethiopathogenesis of Alzheimer’s disease. Although the role of metals as hazardous substances is largely accepted, with even the Agency for Toxic Substances and Disease Registry (ATSDR) including arsenic, lead, mercury, and cadmium in the first ten places on the annual list of hazardous substances,9 much has yet to be clarified as to their impact on neurodegenerative disorders. Some evidence of an association with AD seems to be available only for aluminium. According to a recent metanalysis undertaken on 34 studies involving 1,208 participants, aluminium levels were significantly higher in serum, brain, and the CSF of patients with Alzheimer’s disease.10
The aim of this systematic review is to evaluate the currently available studies concerning metal concentration, except for aluminium, in biological human matrices (blood, serum, hair, brain tissue, nails, cerebrospinal fluid) of AD and non-AD subjects.
Methods
This systematic review was performed according to a-priori protocol, designed following the PRISMA guidelines.11
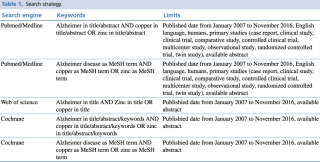
Pubmed/Medline, Scopus and Cochrane databases were examined on 21st November 2016, through a combination of keywords and relevant medical subject heading (MeSH) terms, as extensively shown in Table 1. Search inclusion criteria were restricted to studies on humans, published in the last 10 years, written in English and with an abstract available.
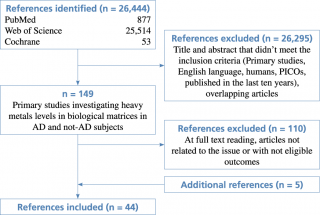
The flow diagram of the complete process selection is shown in Figure 1.
Flow diagram of study selection
Only original studies measuring metal concentrations – except for Aluminium – in biological matrices (blood, urine, hair, nails, cerebrospinal fluid, brain tissue) in AD subjects were considered suitable for inclusion. Cases followed Alzheimer’s disease international diagnostic criteria: NINCDS—ADRDA, DSM-IV, or BRAAK criteria for post-mortem studies.
Four authors (CA, RdD, MM, FP) independently extracted the articles that met the eligibility criteria. Disagreements on the potential relevance of the selected studies were resolved by consensus or by discussion with a fifth author (FL). Furthermore, overlapping articles were excluded.
Additionally, five further works coming from the examination of references of the previews articles were included.16,47,48,52,53
The mean difference in metal concentrations with p value <0.05 was considered as statistically significant result.
A quality assessment of the included studies was performed using the NIH specific tool12 for observational studies evaluation.
Results
Forty-four articles meeting the eligibility criteria were identified: 39 from search engines and 5 additional studies checked from reference lists of the included works. (Table 1; Figure 1).
All the studies were of low to moderate quality, according to the NIH tool score:12 the median score of the selected studies being 5.5 out of 12.
The main characteristics of the included studies are summarized in Table 2-16.
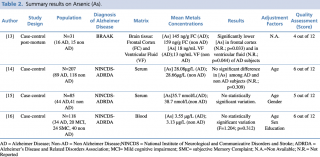
Arsenic (As)
As shown in table 2, an overall number of four studies evaluating arsenic concentrations in biological matrices were considered eligible. Three of the included studies14,15,16 were achieved on serum samples. Park et al. and Paglia et al. found no statistically significant difference (p=0.309 and p=0.312, respectively) in metalloid concentration between AD subjects and healthy controls in two large populations of 207 (89 cases and 118 controls) and 118 subjects (34 AD, 20 MCI, 24 SMC, and 40 non-AD) respectively. A similar result occurred in Baum research: among the 85 enrolled subjects, 44 with a diagnosis of Alzheimer’s disease and 41 healthy controls, there were no relevant differences in arsenic blood concentration between the two groups: a mean level of 35.7 nmol/L in AD subjects compared with 38.7 nmol/L in healthy controls. A post mortem case control study13 carried out by Szabo et al. on 31 brain samples showed significantly lower concentration both in frontal cortex and in ventricular fluid brain regions (p=0.033 and p=0.044, respectively) of AD patients when compared with non-AD subjects (frontal cortex: 145 ng/g vs 159 ng/g; ventricular fluid: 18 ng/ml vs 13 ng/ml, respectively).
Summary results on Arsenic (As)
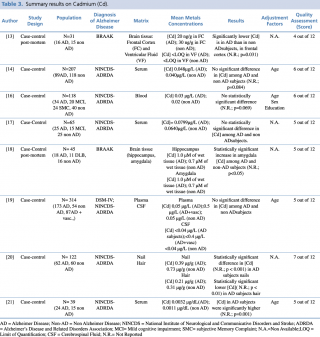
Cadmium (Cd)
Among the five studies assessing cadmium concentration in blood samples of patients with Alzheimer’s disease compared with healthy controls, no significant differences emerged between the two groups (p>0.05). Only one paper21 reported different results, showing a statistically significant (p=0.001) increase in cadmium levels among AD patients. Of note, this study included only 39 subjects, having the smallest size among the considered studies. Only one study19 investigated variations in cadmium cerebrospinal fluid level, showing no statistically significant difference between AD and non AD subjects.
Concerning nails and hair, Koseoglu et al. carried out a case control study on 122 subjects with and without dementia, showing a statistically significant decrease in metal concentration in patients with Alzheimer’s disease compared with healthy controls. Two post mortem studies analysing cadmium levels in brain tissue gave no univocal results. Szabo et al. found a statistically significant (p=0.031) decrease in Cd frontal cortex levels in AD patients, whereas. Akatsu et al. reported a significant (p<0.05) increase in amygdala region of AD compared with non-AD subjects.
Summary results on Cadmium (Cd)
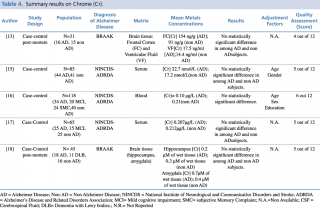
Chrome (Cr)
As shown in Table 4, five articles evaluating a difference in chrome concentration between cases and controls met the eligibility criteria. In 2014 Gonzalez-Dominguez et al. carried out a case-control study to assess serum levels of the metal in 65 subjects: 25 with diagnosis of Alzheimer’s disease, 15 with mild cognitive impairment and 25 healthy patients without dementia. No statistically significant difference in chrome blood concentration was found among the three groups. Similar results were reported by Baum et al., 2010 and by Paglia et al., 2016. Finally, post mortem case control studies by Szabo et al. and Akatsu et al. on brain tissue, found no substantial difference in chrome content, in particular in frontal cortex, ventricular fluid, hippocampus and amygdala.
Summary results on Chrome (Cr)
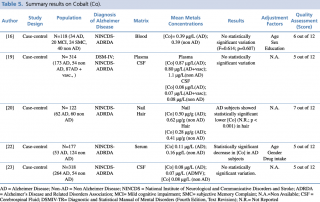
Cobalt (Co)
As shown in Table 5, three studies16,19,22 focused on blood samples (plasma and serum). Alimonti et al. found a significant decrease in serum cobalt among AD subjects in a population of 177 (53 AD and 124 controls), while the studies carried out by Gerhardsson et al. in 2008 and by Paglia et al. in 2016 showed no substantial changes in metal concentration between the two groups. A recent case control study (2017) by Koseoglu et al. focused on Co levels in other biological matrices, hair and nails. The research was undertaken on 122 subjects (62 AD and 60 non-AD) and showed a statistically significant decrease in cobalt concentration (p < 0.001) among AD patients in hair. On the contrary, no real variations emerged by comparison of nail samples between the two investigated groups.
Summary results on Cobalt (Co)
Finally, two studies19,23 assessing metal levels in cerebrospinal fluid on large population samples [N=314 (cases+controls) and N=318 (cases+controls), respectively] did not detect statistically significant differences between cases and controls.
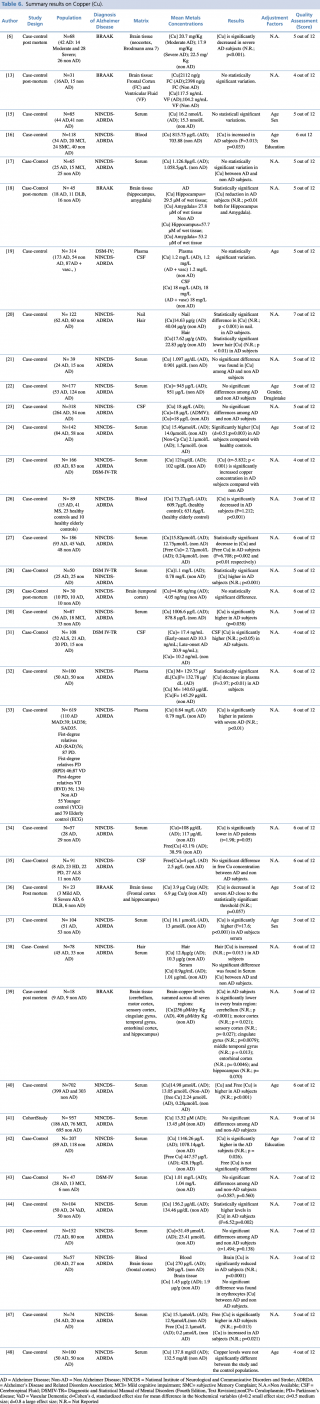
Copper (Cu)
Copper is the most widely investigated metal, with 36 studies assessing its concentrations in different biological matrices (blood, cerebrospinal fluid, hair, nails and brain tissue). As shown in Table 6, most studies assessed copper levels in blood, serum or plasma. The results were extremely variable with 42% of the studies (N=11) showing a significant increase in total and free copper in AD subjects compared with non-AD, 42% (N=11) showing no statistically significant difference between the two groups, and 16% (N=6) showing a significant decrease in AD patients. Also the most robust study in terms of sample population19,33,40,43 gave no univocal results. It must be pointed out that the number of subjects included in studies showing decreased concentrations was generally much lower than in studies showing an increase or no difference. Concerning cerebrospinal fluid, three case control studies19,23,35 found no substantial difference in CSF copper concentration between AD subjects and healthy controls whereas only one study31 showed significantly higher copper levels amongst cases compared with the control group.
Summary results on Copper (Cu)
Few studies have been made on hair or nail matrices. Koseoglu et al.20 evaluated copper levels both in hair and nails on a population of 122 people, reporting a significant decrease in patients with Alzheimer’s disease. On the contrary, Koc et al.,38 assessing metal concentration in hair on a smaller sample (N=78) found no significant difference between AD and non-AD subjects. Finally, a number of post mortem case control studies (N=7), were carried out on brain tissue. More than 70% of eligible articles (5 out of 7)6,18,36,39,41 found a statistically significant decrease in copper brain concentration among AD subjects when compared with healthy controls. Only two studies13,29 showed no relevant variations in metal levels between cases and controls.
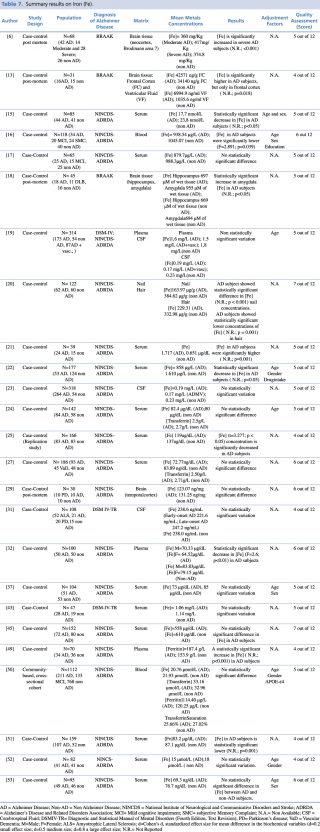
Iron (Fe)
As reported in Table 7, an overall number of 25 articles on iron were considered eligible. Most of the included studies (18 out of 25) were carried out on blood as a biological matrix. One study,50 published in 2014 by Faux et al., was carried out on a large population (N=1,112: 211 with diagnosis of Alzheimer’s disease, 133 with mild cognitive impairment, and 768 healthy controls). In this study there was no evidence of any significant difference in blood iron concentration between AD and non-AD subjects. Non univocal results were reported in the remaining studies. Indeed, ten studies17,19,24,27,37,43,45,50,52,53 didn’t show substantial differences between cases and controls. Six studies15,16,22,25,32,51 showed a significant decrease in blood iron levels of AD patients when compared with healthy controls. Conversely, only one study21 showed a significant increase of iron in AD subjects compared with control group (p=0.001).
Summary results on Iron (Fe)
Concerning hair and nails, a recent study (2017) by Koseoglu et al. gave evidence of a significant decrease of iron levels (p < 0.001 for nails and p=0.001 for hair) among cases when compared to non-AD subjects.
Three further articles investigated cerebrospinal fluid metal concentrations, but with no statistically significant variation between the two groups.
Finally, four post mortem case control studies were performed on different regions of brain tissue and reported discordant results. Szabo et al., Graham et al. and Akatsu et al. found significantly higher iron levels in patients with a diagnosis of Alzheimer’s disease when compared with subjects without dementia. Graham et al. found higher iron levels only in severe AD patients. In Szabo et al.’s work there was an increase in iron concentration in the frontal cortex (p=0.018), but not in the ventricular fluid. The study of Akatsu et al. showed a similar increase in metal but only in the amygdala. The article by Yu et al. showed no statistically significant difference in the temporal cortex region.
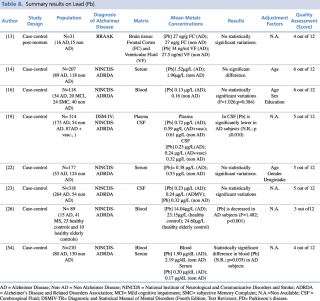
Lead (Pb)
Eight articles concerning lead concentrations in AD and non-AD subjects met the eligibility criteria, as shown in Table 8. Six case control studies, carried out on serum or plasma samples, showed discordant results. Giacoppo et al., in their research on 89 AD and non-AD subjects, found a statistically significant decrease in blood lead levels (p<0.001) in patients with Alzheimer’s disease. The study by Lee et al., carried out in 210 subjects: 80 with a diagnosis of Alzheimer’s disease and 130 without, identified a significant reduction in blood lead concentrations between AD and non-AD subjects (p=0.035). Conversely, the same study also reported an increase in lead levels of patients with Alzheimer’s disease (0.20µg/dL compared with 0.17 µg/dL in the control group) when the evaluation of metal concentration was determined on serum samples.
Summary results on Lead (Pb)
Furthermore, five articles13,14,16,22,23 didn’t report any statistically significant variations in Pb levels between the two groups.
Concerning studies evaluating cerebrospinal fluid samples, similar discordant results were obtained, although on a smaller number of studies.19,23 In their article published in 2009, Gerhardsson et al, evaluating CSF samples in a population of 318 subjects (264 with AD and 54 controls) found no statistically significant difference in metal concentration between AD and non-AD patients. Conversely, the year before another case-control study,18 testing cerebrospinal fluid lead levels in 227 patients, reported a significant reduction (p<0.010) of metal in patients with Alzheimer’s disease compared with healthy controls.
Finally, a recent post mortem case control study, carried out in 2016 by Szabo et al. on brain tissue samples (frontal cortex and ventricular fluid) of 31 subjects, didn’t show any statistically significant differences in Pb levels among AD (N=16) and non-AD subjects (N=15).
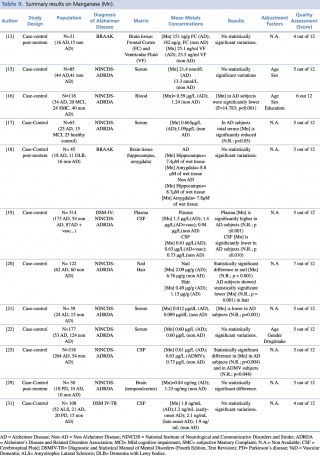
Manganese (Mn)
As shown in Table 9, twelve articles concerning manganese concentration in biological matrices met the eligibility criteria. Six case-controls studies tested differences in blood metal levels, reporting discordant results. Baum et al. and Alimonti et al. didn’t report any significant variations in serum between AD subjects and healthy controls.
Summary results on Manganese (Mn)
Gonzalez-Dominguez et al., compared 25 AD, 15 MCI and 25 non-AD, found significantly lower manganese serum levels (p<0.05) in AD patients when compared with healthy controls. Similar findings were reported by Arslan et al., in a study performed on 39 subjects (24 AD and 15 non-AD), (p=0.001), and by Paglia et al. on 118 subjects (34 AD, 20 MCI, 24 SMC and 40 non-AD) (p <0.001).
By contrast, one study19 showed a significant (p≤0.001) increase in mean plasma concentrations in 173 patients with AD, compared with 54 healthy controls.
Koseoglu et al., in their study on hair and nails samples of 122 subjects, found a significant decrease in metal concentration in both biological matrices among patients with diagnosis of Alzheimer’s disease when compared with non-AD subjects.
Concerning studies on cerebrospinal fluid matrices, Gerhardsson et al. in two articles, published in 2008 and 2009 respectively, reported significantly lower concentrations of manganese in subjects with AD (p ≤0.010 and p=0.004, respectively) than in controls.
Finally, three post-mortem case control study reported no statistically significant difference in Mn brain levels between AD and non-AD subjects, irrespectively from which brain region was investigated (frontal cortex, temporal cortex, hippocampus and amygdala).
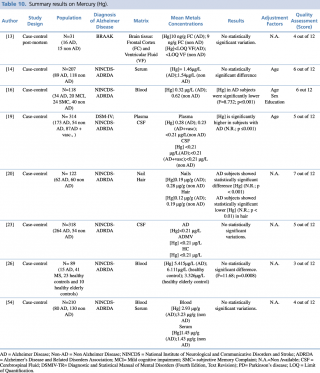
Mercury (Hg)
Eight studies evaluated the difference in mercury concentration between subjects diagnosed with Alzheimer’s disease and healthy patients, as shown in Table 10. Among the five articles assessing blood levels,14,16,19,26,52 only one19 showed statistically significant variations (an increase) in metal levels, while the others found no substantial difference between cases and controls.
Summary results on Mercury (Hg)
Concerning cerebrospinal fluid, Gerhardsson et al. described similar concentrations in cases and controls <0.21 µg/L whereas, a significant decrease in metal concentration in 62 AD patients was reported by Koseoglu et al in comparison to 60 controls.
Finally, a post mortem case control study showed no statistically significant difference in metal concentration of frontal cortex and ventricular fluid between 16 AD and 15 non-AD subjects.
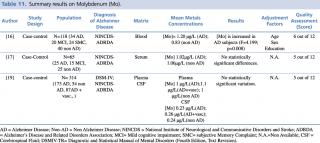
Molybdenum (Mo)
Among the three evaluated articles, only one16 reported an increase (p=0.008) in molybdenum blood concentration in patients with diagnosis of Alzheimer’s disease compared to healthy controls. No statistically significant changes in metal levels were found between cases and controls in the other two reports, irrespectively from the biological matrix investigated (blood, cerebrospinal fluid) (Table 11).
Summary results on Molybdenum (Mo)
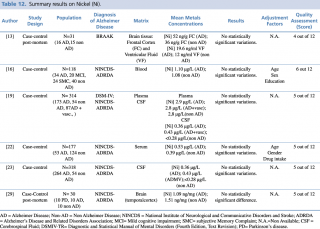
Nickel (Ni)
As shown in Table 12, six studies assessing differences in nickel concentration between AD and non-AD subjects met the eligibility criteria.13,16,19,22,23,29 Independently from the biological matrix evaluated (plasma, serum, cerebrospinal fluid, brain tissue) no significant variation between patients with diagnosis of Alzheimer’s disease and healthy controls was found.
Summary results on Nickel (Ni)
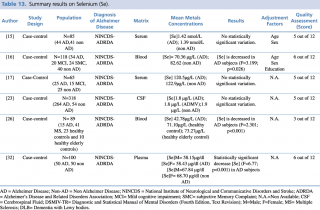
Selenium (Se)
Six articles evaluated selenium levels in patients with diagnosis of Alzheimer’s disease and healthy controls (Table13). Differences in metal concentrations were assessed in two biological matrices: blood and cerebrospinal fluid.
The five studies on blood samples15,16,17,26,32 showed non univocal results: three studies showed a decrease in selenium, whereas no substantial differences were reported in two studies. In detail, Giacoppo et al. reported a significant (p<0.001) decrease in selenium blood levels of 15 AD patients compared with the healthy control group (N=33). A study by Vural et al. found similar results, with blood Se levels that were significantly lower (p<0.001) in patients with Alzheimer’s disease compared to non-AD subjects ([Se]=58.15µg/dl in male and 58.43 µg/dl in female with AD; [Se]=67.84 µg/dl in male and 68.70 µg/dl in female without AD). A study by Paglia et al. found a significant decrease (p= 0.026) of selenium blood levels in AD subjects.
Another two case control studies on selenium blood concentration15,17 showed no substantial variation in metal levels between case and control.
The only available study performed on different biological matrices (Gerhardsson L et al., 2009) found no statistically significant difference between cases and controls in the cerebrospinal fluid samples of 318 subjects (264 AD and 54 healthy controls).
Summary results on Selenium (Se)
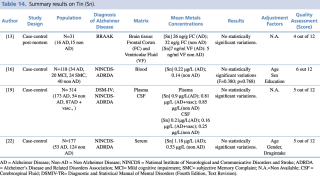
Tin (Sn)
As shown in Table 14, among the four articles that met eligibility criteria, no statistically significant variation in metal levels were found between case and control, irrespective of the biological matrix investigated (blood, brain tissue).
Summary results on Tin (Sn)
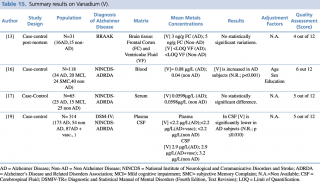
Vanadium (V)
As shown in Table 15, four articles met the eligibility criteria.13,16,17,19 The study by Paglia et al., carried out on 118 subjects (34 AD, 20 MCI, 24 SMC and 40 non-AD), showed a significant decrease (p< 0.001) in vanadium blood levels in AD subjects.
Summary results on Vanadium (V)
By contrast, Gonzalez-Dominguez et al. (studied population: 25 AD, 15 MCI and 25 Healthy Control), and Gerhardsson L et al. (studied population: 173 AD and 54 non-AD patients) found no significant differences among different groups. Conversely from plasma concentrations, Gerhardsson L et al. reported significantly decreased V concentrations in AD patients when compared with healthy controls ([V] =2.9 µg/L in AD versus [V] =3.2 µg/L in non-AD). Furthermore, Szabo ST et al. in their study carried out on brain tissue of 31 subjects (16 AD and 15 non-AD) detected no significant changes in frontal cortex and ventricular fluid vanadium levels.
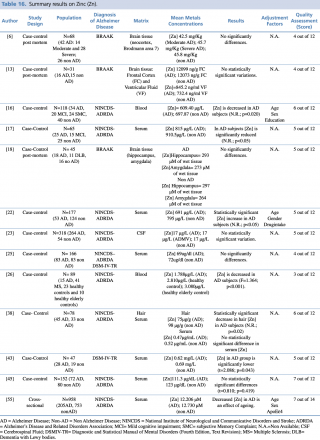
Zinc (Zn)
As shown in Table 16, an overall number of 13 studies assessing zinc concentration in different biological matrices (blood, cerebrospinal fluid, hair, and brain tissue) were eligible. Many of the studies (N=9) evaluated metals levels in serum samples, with not univocal results: 5 studies out of 916,17,26,44,53 showed a significant decrease in zinc blood levels in patients with Alzheimer’s disease; no statistically significant variation in metal concentration emerged from a comparable number of articles.25,38,45 Only the research carried out by Alimonti et al. on 177 subjects resulted in increased levels of zinc among AD subjects when compared with healthy controls. Three further studies were post mortem case controls, undertaken on brain tissue samples, and all showed no significant variations in zinc concentrations between cases and healthy controls. One study19 was carried out on 318 subjects (264 AD and 54 healthy controls) and showed almost identical levels of CSF zinc in cases and controls.
Summary results on Zinc (Zn)
Finally, Koç et al., 2015, in their case control study on a population of 78 subjects found a statistically significant (p=0,02) increase in hair zinc concentration among AD patients compared with healthy controls.
Discussion
Strenghts and limits of the study
Worldwide high incidence and prevalence of Alzheimer’s disease make its prevention and early diagnosis one of the most promising Public Health challenges. Nevertheless, to the best of our knowledge, this is the first systematic study focused on metals as environmental risk factors for Alzheimer’s disease.
Albeit the plethora of researches achieved, demonstrating the clear interest in the topic, mainly poor quality studies are available at the moment. Small sample sizes, no clear choice of exposed and unexposed subjects, high variability of results, lack of adjustment techniques for the most important confounding factors make it often difficult to draw conclusions. Indeed, as reported in Tables 2-16, overall quality of the included studies is quite poor, neither reaching the sufficiency threshold: according to the NIH tool score,12 the median score of the selected studies is 5.5 out of 12.
Furthermore, it is surprising not to find researches investigating interactions among metals, although a tight intercommunication among some of them is well known to influence their absorption, their bond to circulating transporters and regulatory proteins.56
Main findings
The most part of the 44 eligible studies investigating difference in metals concentration reported no univocal outcomes.57 Only some elements gave a clear tendency of results. Chrome, molybdenum, nickel and tin showed no real difference between AD and non-AD subjects, irrespectively to the biological matrix evaluated.
Conversely, a defined tendency to significant variation is available in blood and post-mortem studies investigating copper: blood levels were significantly increased in AD subjects while a decrease in brain tissue concentrations was observed in 70% of the studies, substantially agreeing with results of the metanalysis by Schrag et al.,58 that underlined heterogeneity of the study results on iron, copper and zinc except for brain copper levels, that were significantly depleted in patients with AD. Less robust results were recorded for nail copper levels, that were significantly decreased in patients with Alzheimer’s disease, although this data comes from only one study.
Differently from copper, higher levels of iron were found in the brain tissue of AD subjects, in particular in the Brodmann area, frontal cortex, and amygdala, while there was no significant difference in metal concentration in other biological matrices.
Conclusions
Although much is known about pathogenesis of Alzheimer’s disease, available evidence doesn’t explain all the factors involved in the pathologic process and further models, as “the brain trace metal dyshomeostasis” hypothesis, take place in order to explain those residual part of disease risk. Lifetime absorption of low-moderate metal concentrations, as those recorded in environmental exposure, make it difficult to clearly distinguish between exposed and unexposed subjects.
Besides aluminium, widely recognized as a risk factor for Alzheimer’s disease onset, it has to be pointed out that there is also some evidence of different metal concentrations between AD patients and healthy people, in particular for copper and iron. Concerning the first metal, blood levels are significantly increased in AD subjects, as there is a higher transport towards blood circulation to the detriment of noble organs, such as brain tissue, where copper is decreased, in particular in the hippocampus, amygdala, frontal cortex, cerebellum, motor and sensory cortex, cingulate gyrus, temporal gyrus, and entorhinal cortex. Conversely, in patients with Alzheimer’s disease, higher levels of iron were found in the brain tissue of AD subjects, in particular in the Brodmann area, frontal cortex, and amygdala, while there was no significant difference in metal concentration in other biological matrices. Moreover, because of the complex interaction among iron, copper and zinc, it could be effective to focus on studies investigating Cu/Zn, Cu/Fe, and Zn/Fe ratios as possible biomarkers of effect, instead of concentrating on measuring single metal levels in biological matrices.
Limited evidence was also found on manganese and vanadium: significantly lower blood and cerebrospinal fluid levels were recorded respectively.
Although these hypotheses are fascinating, with the aim to identify groups at risk and to begin to understand Alzheimer’s disease in terms of prevention, interpretation of this study should proceed with caution, in order to avoid speculation.
Indeed, in the environmental field, statistically significant associations do not provide direct evidence of a causal relationship between exposure to metals and Alzheimer’s disease. They trace a direction for future research, reinforcing support to the hypothesis that a dyshomeostasis of metals could be behind the pathogenesis of this disease, also without being its main causal factor.
Therefore, focusing further studies on a limited group of metals, excluding those with a clear tendency to non significant variations in AD subjects (e.g. chrome, molybdenum, nickel and tin), would be a useful approach.
References
- World Alzheimer Report 2015: The Global Impact of Dementia | Alzheimer's Disease International [Internet]. Alz.co.uk. 2017 [cited 26 June 2017]. Available from: https://www.alz.co.uk/research/world-report-2015
- Duthey B. Background paper 6.11. Alzheimer Disease and Other Dementias. 2013. Available from: http://www.who.int/medicines/areas/ priority_medicines/BP6_11Alzheimer.pdf. Accessed June 26, 2017.
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases and Injuries, 1990-2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet. 2016 Oct 8; 388(10053):1545-1602. doi:10.1016/S0140-6736(16)31678-6.
- The Top 10 Causes of Death [Internet]. World Health Organization. 2017 [cited 26 June 2017]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en
- Chen WT, Hong CJ, Lin YT, Chang WH, Huang HT, Liao JY, Chang YJ, Hsieh YF, Cheng CY, Liu HC, Chen YR, Cheng IH. Amyloid-Beta (Ab) D7H Mutation Increases Oligomeric Ab42 and Alters Properties of Ab-Zinc/Copper Assemblies. PLoS ONE; 2012; Volume 7, Issue 4, e35807; 2012
- Graham SF, Nasaruddin MB, Carey M, Holscher C, McGuinness B, Kehoe PG, Love S, Passmore P, Elliott CT, Meharg AA, Green BD. Age-Associated Changes of Brain Copper, Iron and Zinc in Alzheimer’s Disease and Dementia with Lewy Bodies. Journal of Alzheimer’s Disease. J Alzheimers Dis.2014; 42(4):1407-13. doi: 10.3233/JAD-140684; 2014
- Mancinelli M, Lucaroni F, Borgiani P, Ciccacci C, Palombi L, De Filippis P. Low Level Exposure to Arsenic in Drinking Water: A Review on Action Mechanism, Health Effects and Biomarkers. The International Journal of Scientific Research. 2015; 4 (9). Journal DOI: 10.15373/22778179
- Killin LO, Starr JM, Shiue IJ, Russ TC. Environmental Risk Factors for Dementia: A Systematic Review. BMC Geriatr. 2016;16(1):175–175.
- Agency for Toxic Substances and Disease Registry (ATSDR). 2015 Substance Priority List. Available at: https://www.atsdr.cdc.gov/spl/index.html (cited: 11th June 2017)
- Virk Sohaib A. et al. Aluminum and Alzheimer's Disease: A Comprehensive Meta-Analysis. Alzheimer's & Dementia: The Journal of the Alzheimer's Association, 2015; Volume 11, Issue 7, P149 - P150
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ. 2009 Jul 21; 339: b2700. doi: 10.1136/bmj.b2700
- Quality Assessment of Case-Control Studies - NHLBI, NIH [Internet]. Nhlbi.nih.gov. 2017 [cited 4 July 2017]. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in develop/cardiovascular-risk-reduction/tools/case-control
- Szabo S T, Harry G J, Hayden K M, Szabo D T, Birnbaum L. Comparison of Metal Levels Between Postmortem Brain and Ventricular Fluid in Alzheimer's Disease and Nondemented Elderly Controls. Toxicol Sci. 2015; 150, 292–300. 10.1093/toxsci/kfv325
- Park J-H, Lee D-W, Park KS, Joung H. Serum Trace Metal Levels in Alzheimer’s Disease and Normal Control Groups. Am. J. Alzheimers Dis. Other Dement. 2014; 29:76–83. doi: 10.1177/1533317513506778
- Baum L, Chan IH, Cheung SK, Goggins WB, Mok V, Lam L, Leung V, Hui E, Ng C, Woo J, et al. Serum Zinc is Decreased in Alzheimer’s Disease and Serum Arsenic Correlates Positively with Cognitive Ability. Biometals. 2010; 23:173–179. doi: 10.1007/s10534-009-9277-5
- Paglia G, Miedico O, Cristofano A, Vitale M, Angiolillo A, Chiaravalle AE,Corso G, Di Costanzo A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer's Disease. Sci Rep. 2016 Mar 9; 6:22769. doi:10.1038/srep22769
- González-Domínguez R, García-Barrera T, Gómez-ArizaJL. Homeostasis of Metals in the Progression of Alzheimer's Disease. Biometals. 2014 Jun; 27(3):539-49. doi:10.1007/s10534-014-9728-5
- Akatsu H, Hori A, Yamamoto T, Yoshida M, Mimuro M, Hashizume Y, TooyamaI, Yezdimer EM. Transition Metal Abnormalities in Progressive Dementias. Biometals. 2012 Apr; 25(2):337-50. doi: 10.1007/s10534-011-9504-8
- Gerhardsson L, Lundh T, Minthon L, Londos E. Metal Concentrations in Plasma and Cerebrospinal Fluid in Patients with Alzheimer's Disease. Dement GeriatrCognDisord. 2008; 25(6):508-15. doi: 10.1159/000129365
- Koseoglu E, Koseoglu R, Kendirci M, Saraymen R, Saraymen B. Trace Metal Concentrations in Hair and Nails from Alzheimer's Disease Patients: Relations with Clinical Severity. J Trace Elem Med Biol. 2017 Jan; 39:124-128. doi: 10.1016/j.jtemb.2016.09.002
- Arslan A, Tüzün FA, Tamer S, Demir H, Aycan A, Demir C, Tasin M, Gönüllü E, Change of Antioxidant Enzyme Activities, Some Metals and Lipid Peroxidation in Alzheimer’s Disease. ActaMedicaMediterranea, 2016, 32: 1643.doi: 10.19193/0393-6384_2016_5_14
- Alimonti A, Ristori G, Giubilei F, Stazi MA, Pino A, Visconti A, et al. Serum Chemical Elements and Oxidative Status in Alzheimer's Disease, Parkinson Disease and Multiple Sclerosis. NEUROTOXICOLOGY, 2007; 28(3), 450-456
- Gerhardsson L, Blennow K, Lundh T, Londos E, Minthon L. Concentrations of Metals, Beta-Amyloid and Tau-Markers in Cerebrospinal Fluid in Patients with Alzheimer's Disease. Dement GeriatrCognDisord. 2009; 28(1):88-94. doi: 10.1159/000233353
- Siotto M, Simonelli I, Pasqualetti P, Mariani S, Caprara D, BucossiS,Ventriglia M, Molinario R, Antenucci M, Rongioletti M, Rossini PM, Squitti R. Association between Serum Ceruloplasmin Specific Activity and Risk of Alzheimer's Disease. J AlzheimersDis. 2016; 50(4):1181-9. doi: 10.3233/JAD-150611
- Wang ZX, Tan L, Wang HF, Ma J, Liu J, Tan MS, Sun JH, Zhu XC, Jiang T, YuJT. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer's Disease: A Replication Study and Meta-Analyses. J Alzheimers Dis. 2015;47(3):565-81. doi: 10.3233/JAD-143108
- Giacoppo S, Galuppo M, Calabrò RS, D'Aleo G, Marra A, Sessa E, Bua DG, Potortì AG, Dugo G, Bramanti P, Mazzon E. Heavy Metals and Neurodegenerative Diseases: An Observational Study. Biol Trace Elem Res. 2014 Nov;161(2):151-60. doi: 10.1007/s12011-014-0094-5
- Squitti R, Pasqualetti P, Polimanti R, Salustri C, Moffa F, Cassetta E, Lupoi D, Ventriglia M, Cortesi M, Siotto M, Vernieri F, Rossini PM. Metal-Score as a Potential Non-Invasive Diagnostic Test for Alzheimer's Disease. Curr Alzheimer Res. 2013 Feb;10(2):191-8
- Alsadany MA, Shehata HH, Mohamad MI, Mahfouz RG. Histone Deacetylases Enzyme, Copper, and IL-8 Levels in Patients with Alzheimer's Disease. Am J Alzheimers Dis Other Demen. 2013 Feb;28(1):54-61. doi: 10.1177/1533317512467680
- Yu X, Du T, Song N, He Q, Shen Y, Jiang H, Xie J. Decreased Iron Levels in the Temporal Cortex in Postmortem Human Brains with Parkinson Disease. Neurology. 2013 J 29; 80(5):492-5. doi: 10.1212/WNL.0b013e31827f0ebb.
- López N, Tormo C, De Blas I, Llinares I, Alom J. Oxidative Stress in Alzheimer's Disease and Mild Cognitive Impairment with High Sensitivity and Specificity. J Alzheimers Dis. 2013;33(3):823-9. doi: 10.3233/JAD-2012-121528
- Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, Yamada M, Koumura A, Sakurai T, Kimura A, Tanaka Y, Satoh M, Inuzuka T. Patterns of Levels of Biological Metals in CSF Differ Among Neurodegenerative Diseases. J Neurol Sci. 2011 Apr 15;303(1-2):95-9. doi: 10.1016/j.jns.2011.01.003
- Vural H, Demirin H, Kara Y, Eren I, Delibas N. Alterations of Plasmamagnesium, Copper, Zinc, Iron and Selenium Concentrations and Some Related Erythrocyte Antioxidant Enzyme Activities in Patients with Alzheimer's Disease. JTrace Elem Med Biol. 2010 Jul;24(3):169-73. doi: 10.1016/j.jtemb.2010.02.002
- Arnal N, Cristalli DO, de Alaniz MJ, Marra CA. Clinical Utility of Copper, Ceruloplasmin, And Metallothionein Plasma Determinations in Human Neurodegenerative Patients and Their First-Degree Relatives. Brain Res. 2010 Mar 10; 1319:118-30. doi: 0.1016/j.brainres.2009.11.085
- Brewer GJ, Kanzer SH, Zimmerman EA, Celmins DF, Heckman SM, Dick R. Copper and Ceruloplasmin Abnormalities in Alzheimer's Disease. Am J Alzheimers Dis Other Demen. 2010 Sep;25(6):490-7. doi: 10.1177/1533317510375083
- Boll MC, Alcaraz-Zubeldia M, Montes S, Rios C. Free Copper, Ferroxidase and SOD1 Activities, Lipid Peroxidation and NO(X) Content in the CSF. A Different Marker Profile in Four Neurodegenerative Diseases. Neurochem Res. 2008 Sep; 33(9):1717-23. doi: 10.1007/s11064-008-9610-3
- Magaki S, Raghavan R, Mueller C, Oberg KC, Vinters HV, Kirsch WM. Iron, Copper, and Iron Regulatory Protein 2 in Alzheimer's Disease and Related Dementias. Neurosci Lett. 2007 May 11;418(1):72-6
- Squitti R, Ventriglia M, Barbati G, Cassetta E, Ferreri F, Dal Forno G, Ramires S, Zappasodi F, Rossini PM. 'Free' Copper in Serum of Alzheimer's Disease Patients Correlates with Markers of Liver Function. J Neural Transm (Vienna). 2007; 114(12):1589-94. Epub 2007 Jul 4. PubMed PMID: 17641816
- Koç ER, Ilhan A, ZübeydeAytürk, Acar B, Gürler M, Altuntaş A, Karapirli M, Bodur AS. A Comparison of Hair and Serum Trace Elements in Patients with Alzheimer Disease and Healthy Participants. Turk J Med Sci. 2015; 45(5):1034-9
- Xu J, Begley P, Church SJ, Patassini S, McHarg S, Kureishy N, Hollywood KA, Waldvogel HJ, Liu H, Zhang S, Lin W, Herholz K, Turner C, Synek BJ, Curtis MA, Rivers-Auty J, Lawrence CB, Kellett KA, Hooper NM, Vardy ER, Wu D, Unwin RD, Faull RL, Dowsey AW, Cooper GJ. Elevation of Brain Glucose and Polyol-Pathway Intermediates with Accompanying Brain-Copper Deficiency in Patients with Alzheimer's Disease: Metabolic Basis for Dementia. Sci Rep. 2016 Jun 9; 6:27524. doi: 10.1038/srep27524
- Squitti R, Polimanti R, Siotto M, Bucossi S, Ventriglia M, Mariani S, VernieriF, Scrascia F, Trotta L, Rossini PM. ATP7B Variants as Modulators of Copperdyshomeostasis in Alzheimer's Disease. Neuromolecular Med. 2013 Sep;15(3): 515-22.doi: 10.1007/s12017-013-8237-y
- Rembach A, Doecke JD, Roberts BR, Watt AD, Faux NG, Volitakis I, Pertile KK, Rumble RL, Trounson BO, Fowler CJ, Wilson W, Ellis KA, Martins RN, Rowe CC, Villemagne VL, Ames D, Masters CL, AIBL Research Group, Bush AI. Longitudinal Analysis of Serum Copper and Ceruloplasmin in Alzheimer's Disease. J Alzheimers Dis. 2013; 34(1):171-82. doi: 10.3233/JAD-121474
- Park JH, Lee DW, Park KS. Elevated Serum Copper and Ceruloplasmin Levels Inalzheimer's Disease. Asia Pac Psychiatry. 2014 Mar; 6(1):38-45. doi:10.1111/appy.12077
- Huang CW, Wang SJ, Wu SJ, Yang CC, Huang MW, Lin CH, Cheng IH. Potential Blood Biomarker for Disease Severity in the Taiwanese Population with Alzheimer's Disease. Am J Alzheimers Dis Other Demen. 2013 Feb; 28(1):75-83. doi: 10.1177/1533317512467674
- Agarwal R, Kushwaha SS, Tripathi CB, Singh N, Chhillar N. Serum Copper in Alzheimer's Disease and Vascular Dementia. Indian J ClinBiochem. 2008 Oct; 23(4):369-74. doi: 10.1007/s12291-008-0081-8
- Aly WW, Elsaid SMS, Wahba HMF. Copper, Zinc and Iron Serum Levels in Patients with Alzheimer’s Disease. Life Sci J, 2013; 10(3): 2628–2632
- Rembach A., Hare D. J., Lind M., Fowler C. J., Cherny R. A., McLean C., et al. (2013). Decreased Copper in Alzheimer's Disease Brain is Predominantly in the Soluble Extractable Fraction. Int. J. Alzheimers Dis. 2013:623241. 10.1155/2013/623241
- Zappasodi F, Salustri C, Babiloni C, Cassetta E, Del Percio C, ErcolaniM,Rossini PM, Squitti R. An Observational Study on the Influence of Theapoe-Epsilon4 Allele on the Correlation Between 'Free' Copper Toxicosis and EEG Activity in Alzheimer Disease. Brain Res. 2008 Jun 18; 1215:183-9. doi: 10.1016/j.brainres.2008.03.066
- Sedighi B, Shafa MA, Shariati M. A Study of Serum Copper and Ceruloplasmin in Alzheimer’s Disease in Kerman, Iran. Neurology Asia 2006; 11:107-9
- Hare DJ, Doecke JD, Faux NG, Rembach A, Volitakis I, Fowler CJ, Grimm R, Doble PA, Cherny RA, Masters CL, Bush AI, Roberts BR. Decreased Plasma Iron in Alzheimer's Disease is Due to Transferrin Desaturation. ACS ChemNeurosci. 2015 Mar 18; 6(3):398-402. doi: 10.1021/cn5003557
- Faux NG, Rembach A, Wiley J, Ellis KA, Ames D, Fowler CJ, Martins RN, Pertile KK, Rumble RL, Trounson B, Masters CL; AIBL Research Group, Bush AI. An Anemia of Alzheimer's Disease. Mol Psychiatry. 2014 Nov; 19(11):1227-34. doi: 10.1038/mp.2013.178
- Giambattistelli F, Bucossi S, Salustri C, Panetta V, Mariani S, Siotto M, Ventriglia M, Vernieri F, Dell'acqua ML, Cassetta E, Rossini PM, Squitti R. Effects of Hemochromatosis and Transferrin Gene Mutations on Iron Dyshomeostasis, Liver Dysfunction and on the Risk of Alzheimer's Disease. Neurobiol Aging. 2012 Aug; 33(8):1633-41. doi: 10.1016/j.neurobiolaging.2011.03.005
- Torsdottir G, Kristinsson J, Snaedal J, Jóhannesson T. Ceruloplasmin and Iron Proteins in the Serum of Patients with Alzheimer's Disease. Dement GeriatrCognDis Extra. 2011 Jan; 1(1):366-71. doi: 10.1159/000330467
- Squitti R, Salustri C, Siotto M, Ventriglia M, Vernieri F, Lupoi D, CassettaE, Rossini PM. Ceruloplasmin/Transferrin Ratio Changes in Alzheimer's Disease. Int J Alzheimers Dis. 2010 Dec 27; 2011:231595. doi: 10.4061/2011/231595
- Lee JY, Kim JH, Choi DW, Lee DW, Park JH, Yoon HJ, Pyo HS, Kwon HJ, Park KS. The Association of Heavy Metal of Blood and Serum in the Alzheimer's Diseases. Toxicol Res. 2012 Jun; 28(2):93-8. doi: 10.5487/TR.2012.28.2.093
- Rembach A, Hare DJ, Doecke JD, Burnham SC, Volitakis I, Fowler CJ, Cherny RA, McLean C, Grimm R, Martins R, Ames D, Masters CL, Bush AI, Roberts BR. Decreased Serum Zinc Is an Effect of Ageing and Not Alzheimer's Disease. Metallomics. 2014 Jul; 6(7):1216-9. doi: 10.1039/c4mt00060a
- Bjørklund G, Aaseth J, Skalny AV, Suliburska J, Skalnaya MG, Nikonorov AA, Tinkov AA. Interactions of Iron with Manganese, Zinc, Chromium, and Selenium as Related to Prophylaxis and Treatment of Iron Deficiency. J Trace Elem Med Biol. 2017 May; 41:41-53. doi: 10.1016/j.jtemb.2017.02.005. Epub 2017 Feb 12. Review. PubMed PMID: 28347462.
- Mancinelli S, De Filippis P, Messina A, Spoto M, Orlando S, Morciano L, Palombi L and Lucaroni F - Type 2 Diabetes (T2D) and Arsenic at Low Concentrations: Are There Any Real Associations? A Systematic Review. Biomedicine & Prevention (2016) - vol. 1 - (48) - DOI:10.19252/000000030.
- Schrag M, Mueller C, Oyoyo U, Kirsch WM. Iron, Zinc and Copper in the Alzheimer’s Disease Brain: A Quantitative Meta-Analysis. Some Insight on the Influence of Citation Bias on Scientific Opinion. Progress in Neurobiology. 2011; 94(3):296-306. doi:10.1016/j.pneurobio.2011.05.001.