Autism Spectrum Disorder in Tuberous Sclerosis: The preventive value of early detection
Arianna Benvenuto,1 Martina Siracusano,1 Federica Graziola,1 Sara Marciano,1 Leonardo Emberti Gialloreti,2,3 Luigi Mazzone,1,4 Romina Moavero,1,5 Paolo Curatolo1
1Child Neurology and Psychiatry Unit, System Medicine Department, Tor Vergata University Hospital of Rome, Italy
2Department of Biomedicine and Prevention, University of Rome, Tor Vergata University of Rome, Italy
3Centre for Communication and Neurorehabilitation Research, CNAPP, Rome, Italy
4Child Psychiatry Unit, Department of Neuroscience and Neurorehabilitation, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
5Child Neurology Unit, Department of Neuroscience and Neurorehabilitation, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
Introduction
Tuberous Sclerosis Complex (TSC, MIM#191100) is a multisystem genetic disorder that affects many organs and systems, with an estimated birth incidence of 1:5,800.1 Symptoms and severity vary widely between individuals with hamartomatous lesions affecting various organs including brain, skin, kidneys, heart and eyes.2
The disorder is caused by an autosomal dominant mutation in either the TSC1 gene3 on chromosome 9q34 or the TSC2 gene on chromosome 16p13.3,4 which code respectively for hamartin (130KDa) and tuberin (200KDa). The TSC1-TSC2 protein complex indirectly inhibits a downstream signaling pathway, the mammalian target of rapamycin (mTOR). As a result of either gene mutation, mTOR overactivation may produce disrupted synaptic plasticity, reduced autophagy, abnormal neuronal morphology, and abnormal cell growth and proliferations.5
The Central Nervous System (CNS) is affected in more than 90% of individuals with TSC; pathological lesions include cortical and subcortical tubers, subependymal nodules, giant cells astrocytomas and white matter migration lines, which may alter various aspects of intracellular functions and may produce neurological signs and symptoms such as epilepsy and neuropsychiatric disorders.2
TSC is associated with a wide range of neuropsychiatric manifestations, including intellectual disability (ID), autism spectrum disorder (ASD), and attention deficit hyperactivity disorder (ADHD).2 About 50% of individuals present normal intellectual ability, while the remaining half has different level of cognitive impairment. Epidemiological findings suggest an over representation of severe and profound intellectual disability in TSC.6
TSC represents one of the syndromic forms most strongly associated with a high prevalence of ASD, together with Fragile X syndrome.7, 8 However, despite recent advances in molecular genetics, the underlying causes of ASD remain largely unknown. Nevertheless, the recent identification of monogenic forms of ASD, like TSC, has provided new insights for the identification of treatment targets; clinical trials with syndromic forms of ASD are now ongoing.
ASD is a lifelong condition with an early onset characterized by persistent deficits in social communication, as well as restricted and repetitive patterns of behavior.9 The co-occurrence of ASD and TSC has long been recognized, and autistic-like symptoms were first observed by Critchley and Earl in 1932 who described 29 individuals with impaired social contact, repetitive and stereotyped behaviour, absent or abnormal speech and social withdrawal.10 The presence of autistic traits in infants with TSC can be evident even within the first 12 months of life; most of these children show a specific deficit in play that is evident from early infancy, associated with impairment in communication and social interaction, and poor quality of eye contact.8, 11 After the second year of life, other behavioral problems such as overactivity, repetitive and ritualistic behaviors, and temper tantrums, become evident.8
Despite studying different TSC populations with different geno-phenotype profile, all the epidemiological studies of ASD-TSC revealed a surprising high incidence of ASD, which ranged between 17% and 63%.12 Numis et al., in a single cohort of 103 patients evaluated by a single neuropsychologist, found a 40% ASD prevalence.13 Although it is well established that ASD is much more frequent in individuals with TSC than in the general population, the underlining reason of this association remains largely unclear. Moreover, despite TSC is increasingly diagnosed early in development, only few studies prospectively evaluated the early onset of ASD and developmental delay in infants with TSC.11,14
Nevertheless, the epidemiological picture clearly shows that TSC is a weighty risk factor for the development of ASD. Consequently, it would be useful also to understand which subjects with TSC present with the highest risk of developing ASD, so to identify possible strategies to prevent or to shrink some of the future symptoms of ASD and/or autistic like behaviors.
As already highlighted, due to complexity of the association between TSC and ASD and the still limited understanding of their multiple risk factors, primary prevention interventions are not yet feasible. However, the identification of early or very early signs and phenotypes might become an important secondary prevention tool, with the aim to reduce and modify the impact of the disease. In TSC associated ASD, this can be done by detecting and intervening as soon as possible to modify the developmental trajectory of the child and/or to prevent long-term adverse outcomes. Aim of this paper is to discuss potential factors for an increased risk of ASD in TSC, and possible secondary prevention strategies in ASD associated with TSC.
Potential Factors for an Increased Risk of ASD in TSC
Potential factors of a clinical association between ASD and TSC include genetic mutations, structural brain abnormalities and epilepsy.
Genetic mutations
In mouse models/preclinical studies, TSC2 mutations cause more severe histologic abnormalities and more severe neurological phenotypes than TSC1 mutations.15 Recent evidence and genotype – phenotype studies have shown that some genetic mutations may cause a higher risk to develop ASD. Patients with ASD in comparison with patients without ASD were significantly more likely to have TSC2 mutations.13
A specific relation with autism in mutations occurring in the hamartin interaction domain of TSC2 has been reported as well.13
Structural brain abnormalities
In early studies, the high comorbidity of ASD and TSC had been suggested by the presence of tubers localized in temporal lobes.16 A higher tuber number has been described in patients with TSC2 mutations.17 However, early attempts to differentiate TSC children with or without autism on the basis of structural cortical-subcortical tubers led to inconsistent results. More recently, several attempts have been made to differentiate these two groups on the basis of white matter functional integrity, which has been analyzed by means of Diffusion Tensor Imaging (DTI). Abnormal connectivity in the corpus callosum and the arcuate fasciculus have been observed trough structural whole-brain connectivity maps.18
Furthermore, tuber brain proportion and/or white matter abnormalities have been identified as potential risk factor for poor cognitive outcome for ASD.19,20 Widespread DTI abnormalities have been detected in children with ASD and TSC compared to children with TSC without ASD, suggesting microstructural changes in myelination, axonal integrity, or extracellular environment.18 Lastly, cyst-like tubers have been reported to be more numerous in children with than without ASD.13
Seizures
Epilepsy affects up to 85% of the individuals with TSC, being much more frequent and more refractory in patients with the TSC2 mutation. Early age onset seizures, infantile spasms and the persistence of severe seizures have been commonly identified as potential risk factors for autism, with early age at seizure onset being the only independent risk factor for adverse neurodevelopmental outcome.8,20 In a cohort of 103 TSC patients, earlier age onset seizure, higher frequency and refractory seizures, and a greater amount of interictal epileptiform abnormalities in the left temporal lobe were associated with higher risk of autism.13
Mechanisms of actions may be various. Firstly, the electrophysiological disturbances may interfere with activity dependent mechanisms involved in synaptogenesis and dendritic arborisations, and may disrupt the proper establishment of social cognitive representation in the left temporal region.21 Interestingly, persistent seizure activity might disrupt brain connectivity in areas crucial for the normal development of cognitive and emotional functions, including the cingulum and temporal trunk.22 Finally, the timing of seizure onset and the frequency of seizures are associated with ASD, where seizures occurring in early developmental stage, – during the critical period of synaptogenesis – activate mTORC1, contributing to epileptic networks and autistic-like behaviors in later life.23
The mTOR signaling pathway plays a role in regulating numerous cellular processes in the developing brain, including neuronal cell morphology GABAergic interneurons development, white matter connectivity, number and shape of synapses.24 Therefore, the final common pathway in the development of an autistic brain may be a neuronal connectivity perturbation in which abnormalities of local network information processing can interfere with the development of appropriate long-range connections among affected brain regions.25 Altered interneuron development, including a disruption in GABAergic interneurons, may account for an abnormal balance of excitation and inhibition that can explain the concomitant presence of epilepsy and ASD in infants with TSC.
Early Detection of Autistic Traits and Relationship with Developmental Impairment in TSC
Early age deficits in visual reception and fine motor ability at age of 6 months might predict future deviations of the developmental trajectories.14 Also the use of clinical instruments for ASD screening, such as the Autism Observation Scale for Infants (AOSI), by the age of 6 months, demonstrated more atypical social behaviors in visual tracking, disengagement of attention, and anticipatory responses. By the age of 9 and 12 months, abnormalities were also observed in eye contact, orienting to name, motor control and behavior. The early delay in the visual domain leads to difficult early visual perception, placing these infants at higher risk of deficits in social function. The visual impairment might be explained by the TSC mouse model, where there is evidence of aberrant structural connectivity in visual projections, which appear more diffuse and less organized.26 This early sign seems to be specific of the TSC population and differ from the other “red flags” identified for idiopathic forms of ASD, such as social babbling or orienting to name.
Children with TSC and ASD present a more significant developmental delay than those with TSC without ASD.11,14 In particular, in both 24- and 36-month-old children, those with autism had the lowest scores in all developmental quotients, those diagnosed with ASD had scores in the middle of the range, whereas those without autism/ASD had the highest scores.11,14 At 12 months, the association between ASD and developmental impairment reached statistical significance for visual reception and receptive and expressive language, and these results were confirmed also at the 36 months follow-up.11,14
The developmental trajectories of infants with TSC seem to be strictly correlated with ASD diagnosis. In fact, ASD patients showed no change in full developmental quotient between 2 and 3 years, but they experienced a significant decline in non-verbal quotient between 12 and 36 months; on the other hand, the non-ASD group showed significant developmental gains in the non-verbal quotient, but not in verbal abilities.14 Finally, the majority of children with TSC, regardless of their diagnosis of autism/ASD, showed impairments in playing, thus suggesting a common developmental deficit in all children with TSC, with a wide spectrum of severity that determines the diagnosis of autism/ASD.11,14
Preventive Potential of Early Interventions
Early interventions
Despite the fact that infants with TSC have a strong neurobiological basis for their abnormal developmental trajectories and have a higher susceptibility for autism, this doesn't preclude the potential efficacy of an early intervention far before the age of 24 months. Actually, today it is possible to identify early deviations from the expected developmental trajectories in some subgroups of high risk infants.
The importance of an early identification of ASD in children under the age of 2 years is crucial to initiate an early treatment that could ameliorate its disabling effects by making use of the greater brain plasticity of the early ages. Research studies in animal models of syndromic ASD revealed that an enhanced environmental stimulations could ameliorate the effects of a wide range of neurological challenges.27,28 Therefore, it is essential to identify predictive preclinical markers in infants with TSC aged 6-12 months.
Treatment strategies should be individualized and targeted at improving social communication function as well as broader developmental domains associated with ASD, before the onset of autism symptoms. In this view, recent models of the early emergence of autistic signs in children at risk for ASD propose that early intervention designed to promote early social engagement and reciprocity could potentially remit or reduce the expression of symptoms in several high-risk populations.29
A number of prospective studies of infant siblings of ASD children have also demonstrated that improvements in parent-infant synchrony could hypothetically lead to improvements in child dyadic communication and reductions in ASD symptoms.30,31 Parent-infant relationship-focused intervention, aimed at eliciting more frequent functional communication and relationship and stimulating social brain circuitry, could be a good model of treatment also for children with TSC. For all these reasons, clinical trials on prospective neurodevelopmental assessment and more targeted interventions for these high-risk infants are warranted.
Targeted treatments
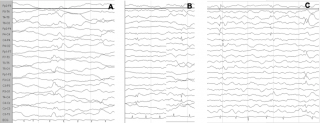
Among all the mentioned factors that increase the risk for ASD in TSC, only seizures might be potentially preventable, and it is believable that early detection and treatment of both epileptiform abnormalities and clinical seizures in TSC could change the course of the disorder. In a growing number of pregnancies, prenatal diagnosis of TSC is possible by detecting cardiac rhabdomyomas and cortical tubers; in these cases it is therefore possible to identify infants at high risk of severe epilepsy and/or ASD.2 Seizures are preceded by a latent period of epileptogenesis, during which epileptiform abnormalities might appear in the EEG32,33 (Figure 1). Early detection and prompt control of seizures and epileptiform EEG abnormalities play a crucial role in preventing subsequent epileptic encephalopathy and in reducing the cognitive and behavioural consequences of epilepsy.34
Figure 1: Evolution of the EEG pattern at 3, 4 and 5 months of age (a, b, c), which shows the tendency of epileptiform abnormalities to become multifocal.
Vigabatrin is known to be particularly beneficial in TSC associated infantile spasms,35 and this specific efficacy might be due not only to the GABAergic effect but also – at least in part – to mTOR inhibiting activities.36 A shorter gap between seizure onset and start of treatment with vigabatrin could reduce the risk of epileptic encephalopathy and the appearance of profound ID and ASD, while minimizing the effects of early life seizures. However, antiepileptic treatment given during the period of epileptogenesis and before any clinical detectable seizures could show even a better efficacy,37 with a high ratio of patients achieving seizure freedom and fewer children with profound ID and severe ASD. However, the optimal timing for administering vigabatrin is still matter of debate, and a multicentre study aimed to answer this question is currently ongoing (EPISTOP: Long-term, Prospective Study Evaluating Clinical and Molecular Biomarkers of Epileptogenesis in a Genetic Model of Epilepsy – Tuberous Sclerosis Complex – NCT02098759).
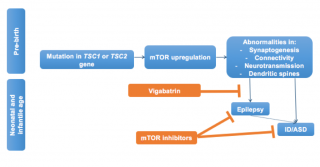
In Figure 2 we illustrate the possible cascade of events generated by the mutation of one of the two genes leading to mTOR activation, and the pre- and post-epileptic window for secondary prevention of seizures and autism in infants with TSC. New research is showing that mTORC1 signaling is already activated in various brain cell populations, including dysmorphic neurons within the dysplastic cortex and giant cells within the subcortical white matter in foetal tubers.38 This mTOR over-activity causes the possible underlying pathologic substrate for seizures, TSC-related seizures, and encephalopathy. Different windows of opportunity for the prevention of the development of seizures and associated neurobehavioural disorders is therefore possible.
Figure 2: Summary of the cascade of events resulting from the mutation of the TSC1/2 genes and taking place already during fetal life. Poorly controlled seizures are significant risk factors for adverse neurobehavioral and neurocognitive outcomes. Pre and post epileptic windows for secondary prevention are theoretically possible in infants with TSC. The early treatment with vigabatrin might reduce or suppress seizures thus decreasing the risk of subsequent ID/ASD; while mTOR inhibitors might directly act on both seizures and behavioral phenotypes.
Rapamycin, a selective inhibitor of mTOR complex, reversed learning deficit in a Tsc2+/- mouse, even when administered in adulthood. A subsequent study on a mouse model of early onset epilepsy and autism, demonstrated that rapamycin was able not only to reduce seizure susceptibility, but also to attenuate autistic-like behaviours.23,24 There is also some preliminary clinical evidence of beneficial effects of mTOR inhibitors, including rapamycin and its analogue everolimus, on autistic symptoms in a few patients.39 Several clinical trials are already in progress to investigate neurocognitive disorders and autism in TSC, as well as the possible efficacy and safety of mTOR inhibitors (Efficacy of RAD001/Everolimus in Autism and NeuroPsychological Deficits in Children With Tuberous Sclerosis Complex (RAPIT) – NCT01730209; Rapalogues for Autism Phenotype in TSC: A Feasibility Study (RAPT) – NCT01929642; Trial of RAD001 and Neurocognition in Tuberous Sclerosis Complex – NCT01289912). There is a critical need to evaluate the efficacy of mTOR inhibition in infants and young children with TSC for the assessment of optimal developmental neurocognitive and behavioural outcomes. Both basic and clinical science evidence thus suggest that mTOR inhibitors could be a rational candidate for preventing the development of these specific disorders.40
Future Perspectives for Prevention and Treatment of ASD in TSC
As described, mTOR dysregulation could play a direct role in determining susceptibility to ASD, cognitive impairment, and epilepsy. Seizures could have an addictive effect, increasing the likelihood of the development of autistic like behaviours, even if the TSC1/2 gene mutation may be sufficient to lead to social deficits.41 A better understanding of the early biomarkers of developmental outcome can give us a specific therapeutic window in which early targeted treatment could obtain better benefits, thus maximizing their efficacy. An early use of vigabatrin has been found to be associated with a significant reduction of neurobehavioral symptoms later in life, but it is not able to fully revert the outcomes. The results of the ongoing multicenter study EPISTOP will give us some more information on the optimal timing for initiating treatment in high risk infants, and to understand if a preventative treatment can – at least in part – modify the natural history of the disease. Preventative trials with mTOR inhibition as antiepileptogenic treatment could be now designed in pre-symptomatic infants under the age of 24 months to evaluate if this strategy could have preventative or disease modifying effects, prolonging seizure freedom, inhibiting evolution toward an epileptic encephalopathy, and preventing or mitigating intellectual disability and ASD symptomatology.
Acknowledgments
A. Benvenuto, R. Moavero and P. Curatolo participate in the EPISTOP study (www.epistop.eu) which is funded under the European Community's Seventh Framework Programme (FP7/2007- 2013) under Grant Agreement n 602391.
References
- O’Callaghan FJ, Shiell AW, Osborne JP, Martyn CN. Prevalence of Tuberous Sclerosis Estimated by Capture-Recapture Analysis. Lancet. 1998;351(9114):1490.
- Curatolo P, Moavero R, de Vries PJ. Neurological and Neuropsychiatric Aspects of Tuberous Sclerosis Complex. Lancet Neurol. 2015;14(7):733-45.
- Van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, et al. Identification of the Tuberous Sclerosis Gene TSC1 on Chromosome 9q34. Science. 1997;277(5327):805-8.
- European Chromosome 16 Tuberous Sclerosis C. Identification and Characterization of the Tuberous Sclerosis Gene on Chromosome 16. Cell. 1993;75(7):1305-15.
- Napolioni V, Moavero R, Curatolo P. Recent Advances in Neurobiology of Tuberous Sclerosis Complex. Brain Dev. 2009;31(2):104-13.
- Joinson C, O’Callaghan FJ, Osborne JP, Martyn C, Harris T, Bolton PF. Learning Disability and Epilepsy in an Epidemiological Sample of Individuals with Tuberous Sclerosis Complex. Psychological Medicine. 2003;33(2):335-44.
- Benvenuto A, Moavero R, Alessandrelli R, Manzi B, Curatolo P. Syndromic Autism: Causes and Pathogenetic Pathways. World Journal of Pediatrics. 2009;5(3):169-76.
- Curatolo P, Napolioni V, Moavero R. Autism Spectrum Disorders in Tuberous Sclerosis: Pathogenetic Pathways and Implications for Treatment. J Child Neurol. 2010;25(7):873-80.
- AmericanPsychiatricAssociation. Diagnostic and Statistical Manual of Mental Disorders. 4th edition ed. Association AP, editor. Washington DC, USA1994. 886 p.
- Critchley M, Earl C. Tuberous Sclerosis and Allied Conditions. Brain. 1932;55:311-46.
- Jeste SS, Sahin M, Bolton P, Ploubidis GB, Humphrey A. Characterization of Autism in Young Children with Tuberous Sclerosis Complex. J Child Neurol. 2008;23(5):520-5.
- Wong V. Study of the Relationship between Tuberous Sclerosis Complex and Autistic Disorder. J Child Neurol. 2006;21(3):199-204.
- Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB, Thiele EA. Identification of Risk Factors for Autism Spectrum Disorders in Tuberous Sclerosis Complex. Neurology. 2011;76(11):981-7.
- Spurling Jeste S, Wu JY, Senturk D, Varcin K, Ko J, McCarthy B, et al. Early Developmental Trajectories Associated with ASD in Infants with Tuberous Sclerosis Complex. Neurology. 2014;83(2):160-8.
- Zeng LH, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M. Tsc2 Gene Inactivation Causes a More Severe Epilepsy Phenotype than Tsc1 Inactivation in a Mouse Model of Tuberous Sclerosis Complex. Hum Mol Genet. 2011;20(3):445-54.
- Bolton PF, Park RJ, Higgins JN, Griffiths PD, Pickles A. Neuro-Epileptic Determinants of Autism Spectrum Disorders in Tuberous Sclerosis Complex. Brain. 2002;125(Pt 6):1247-55.
- Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, et al. Mutational Analysis in a Cohort of 224 Tuberous Sclerosis Patients Indicates Increased Severity of TSC2, compared with TSC1, Disease in Multiple Organs. Am J Hum Genet. 2001;68(1):64-80.
- Peters JM, Sahin M, Vogel-Farley VK, Jeste SS, Nelson CA, 3rd, Gregas MC, et al. Loss of White Matter Microstructural Integrity is Associated with Adverse Neurological Outcome in Tuberous Sclerosis Complex. Academic Radiology. 2012;19(1):17-25.
- Chou IJ, Lin KL, Wong AM, Wang HS, Chou ML, Hung PC, et al. Neuroimaging Correlation with Neurological Severity in Tuberous Sclerosis Complex. European Journal of Pediatric Neurology: EJPN. 2008;12(2):108-12.
- Jansen FE, Vincken KL, Algra A, Anbeek P, Braams O, Nellist M, et al. Cognitive Impairment in Tuberous Sclerosis Complex Is a Multifactorial Condition. Neurology. 2008;70(12):916-23.
- Holmes GL, Ben-Ari Y. The Neurobiology and Consequences of Epilepsy in the Developing brain. Pediatric Research. 2001;49(3):320-5.
- Moavero R, Napolitano A, Cusmai R, Vigevano F, Figa-Talamanca L, Calbi G, et al. White Matter Disruption Is Associated with Persistent Seizures in Tuberous Sclerosis Complex. Epilepsy Behav. 2016;60:63-7.
- Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, et al. The Interaction between Early Life Epilepsy and Autistic-Like Behavioral Consequences: A Role for the Mammalian Target of Rapamycin (mTOR) Pathway. PLoS One. 2012;7(5):e35885.
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, et al. Reversal of Learning Deficits in a Tsc2+/- mouse Model of Tuberous Sclerosis. Nature Medicine. 2008;14(8):843-8.
- Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic Disruption of Cortical Interneuron Development Causes Region- and GABA Cell Type-Specific Deficits, Epilepsy, and Behavioral Dysfunction. J Neurosci. 2003;23(2):622-31.
- Nie D, Di Nardo A, Han JM, Baharanyi H, Kramvis I, Huynh T, et al. Tsc2-Rheb Signaling Regulates EphA-Mediated Axon Guidance. Nature Neuroscience. 2010;13(2):163-72.
- Favre MR, La Mendola D, Meystre J, Christodoulou D, Cochrane MJ, Markram H, et al. Predictable Enriched Environment Prevents Development of Hyper-Emotionality in the VPA Rat Model of Autism. Frontiers in Neuroscience. 2015;9:127.
- Woo CC, Donnelly JH, Steinberg-Epstein R, Leon M. Environmental Enrichment as a Therapy for Autism: A Clinical Trial Replication and Extension. Behavioral Neuroscience. 2015;129(4):412-22.
- Koegel L, Singh A, Koegel R, Hollingsworth J, Bradshaw J. Assessing and Improving Early Social Engagement in Infants. Journal of Positive Behavior Interventions. 2014;16(2):69-80.
- Green J, Charman T, McConachie H, Aldred C, Slonims V, Howlin P, et al. Parent-Mediated Communication-Focused Treatment in Children with Autism (PACT): A Randomized Controlled Trial. Lancet. 2010;375(9732):2152-60.
- Koegel LK, Koegel RL, Ashbaugh K, Bradshaw J. The Importance of Early Identification and Intervention for Children with or at Risk for Autism Spectrum Disorders. International Journal of Speech-Language Pathology. 2014;16(1):50-6.
- Rakhade SN, Jensen FE. Epileptogenesis in the Immature Brain: Emerging Mechanisms. Nature Reviews Neurology. 2009;5(7):380-91.
- Pitkanen A, Lukasiuk K. Mechanisms of Epileptogenesis and Potential Treatment Targets. Lancet Neurol. 2011;10(2):173-86.
- Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early Control of Seizures Improves Long-Term Outcome in Children with Tuberous Sclerosis Complex. European Journal of Paediatric Neurology : EJPN. 2010;14(2):146-9.
- Chiron C, Dumas C, Jambaque I, Mumford J, Dulac O. Randomized Trial Comparing Vigabatrin and Hydrocortisone in Infantile Spasms Due to Tuberous Sclerosis. Epilepsy Res. 1997;26(2):389-95.
- Zhang B, McDaniel SS, Rensing NR, Wong M. Vigabatrin Inhibits Seizures and mTOR Pathway Activation in a Mouse Model of Tuberous Sclerosis Complex. PLoS One. 2013;8(2):e57445.
- Jozwiak S, Kotulska K, Domanska-Pakiela D, Lojszczyk B, Syczewska M, Chmielewski D, et al. Antiepileptic Treatment before the Onset of Seizures Reduces Epilepsy Severity and Risk of Mental Retardation in Infants with Tuberous Sclerosis Complex. European Journal of Pediatric Neurology: EJPN. 2011;15(5):424-31.
- Prabowo AS, Anink JJ, Lammens M, Nellist M, van den Ouweland AM, Adle-Biassette H, et al. Fetal Brain Lesions in Tuberous Sclerosis Complex: TORC1 Activation and Inflammation. Brain Pathology. 2013;23(1):45-59.
- Kilincaslan A, Kok BE, Tekturk P, Yalcinkaya C, Ozkara C, Yapici Z. Beneficial Effects of Everolimus on Autism and Attention-Deficit/Hyperactivity Disorder Symptoms in a Group of Patients with Tuberous Sclerosis Complex. J Child Adolesc Psychopharmacol. 2016.
- Davis PE, Peters JM, Krueger DA, Sahin M. Tuberous Sclerosis: A New Frontier in Targeted Treatment of Autism. Neurotherapeutics. 2015;12(3):572-83.
- Waltereit R, Japs B, Schneider M, de Vries PJ, Bartsch D. Epilepsy and Tsc2 Haploinsufficiency Lead to Autistic-Like Social Deficit Behaviors in Rats. Behavior Genetics. 2011;41(3):364-72.